Suppression of Protein Kinase C-ÃÆà ½Ãâñ Ameliorates Hyperglycaemia-Evoked In Vitro Cerebral Barrier Dysfunction
Kirtiman Srivastava, Ulvi Bayraktutan
1Stroke, Division of Clinical Neuroscience, University of Nottingham, Nottingham, UK
2Centre for Cancer Research and Cell Biology, Queen’s University Belfast,Belfast, UK
- *Corresponding Author:
- Dr Ulvi Bayraktutan
Stroke, Division of Clinical Neuroscience
Clinical Sciences Building, Nottingham City Hospital Campus,
The University of Nottingham, Hucknall Road, Nottingham, UK
Tel: +44(115)8231764
Fax: +44(115)8231767
E-mail: ulvi.bayraktutan@nottingham.ac.uk
Received Date: March 20, 2017; Accepted Date: April 13, 2017; Published Date: April 18, 2017
Citation: Srivastava K, Bayraktutan U. Suppression of Protein Kinase C-α Ameliorates Hyperglycaemia-Evoked In Vitro Cerebral Barrier Dysfunction. Stroke Res Ther. 2017, 2:1.
Abstract
Title: Suppression of protein kinase C-α ameliorates hyperglycaemia-evoked in vitro cerebral barrier dysfunction.
Background: Ischaemic stroke is common in diabetes patients and occurs due to cerebral-barrier dysfunction. Although the pathological activation of protein kinase C (PKC) is linked with the disease progression, the PKC isoform-specific downstream signalling remains obscure and is the focus of this study which explored the clinical relevance of PKC-α inhibitor Ro-32-0432 under hyperglycaemia (HG).
Methods and Findings: Total PKC and RhoA activities were studied in human brain microvascular endothelial cells (HBMEC) exposed to normoglycaemia or HG. The integrity and function of an in vitro model of human BBB composed of HBMEC and human astrocytes were measured by transendothelial electrical resistance (TEER) and paracellular flux of Evans blue-labelled albumin (EBA), respectively. Exposure of HBMEC to HG for 72 hours led to significant increases in activities of total PKC and RhoA as well as in mono- and di-phosphorylation of MLC2 which concurred with substantial decreases in TEER and marked elevations in barrier permeability. Enhanced cellular contractility triggered by actin stress fibre formation appeared to further potentiate HG-mediated barrier dysfunction. Pre-treatment of HBMEC with Ro-32-0432 or transient PKC-α protein knockdown led to effective preservation of BBB integrity and function in hyperglycaemic settings by suppressing RhoA activity and subsequently normalising the MLC2 phosphorylation on Ser19 and Thr18- Ser19 residues. These observations were further supported by disappearance of stress fibres and subsequent restoration of the cortical actin staining.
Conclusions: Neutralisation of PKC-α activity may be of considerable therapeutic value in clinical settings accompanied by diabetes or stress HG.
Keywords
Endothelial cell; Hyperglycaemia; Protein kinase C; Ro-32-0432; RhoA; cytoskeletal remodeling
Introduction
The vascular endothelium lines the entire inner surface of all blood vessels and forms specific barriers in different organs to regulate vascular permeability. The blood-brain barrier (BBB) represents one such barrier and prevents the leakage of bloodborne substances into brain parenchyma. Disruption of the BBB characterised by the formation of brain oedema is a common occurrence after ischaemic strokes and constitutes the main cause of mortality within the first week after a cerebrovascular attack. As the brain oedema is more prevalent in stroke patients with diabetes mellitus, it is thought that hyperglycaemia may be intricately involved in the pathogenesis of this defect [1-3].
The BBB consists of brain microvascular endothelial cells (BMEC), capillary basement membrane and astrocyte end-feet supporting the basement membrane. The permeability of the BBB is tightly regulated by tight junction proteins, notably occludin and claudin-5, that exist between the adjacent BMEC. Hence, pathologies that affect BMEC phenotype and/or tight junction protein expression and localisation would have an adverse effect on the integrity and function of the BBB. Although several biochemical pathways including non-enzymatic glycation of proteins and lipids with the irreversible deposition of advanced glycation end products, the activation of polyol pathway or stimulation of protein kinase C (PKC) pathway have been implicated in diabetes-mediated peripheral vasculopathies, the mechanisms involved in hyperglycaemia-evoked cerebrovascular impairments remain largely unknown [4].
In addition to regulation of PKC activity, the suppression of RhoA may also be an efficacious therapeutic option in protecting cerebral barrier integrity given the fact that binding of active RhoA to its downstream effector Rho-kinase leads to the disruption of intercellular junctions by sequential induction of myosin-regulatory light chain-2 phosphorylation (pMLC2) and actin stress fibre formation [5-16].
In light of the above, this study investigated the specific role of PKC-α isoform in hyperglycaemia-mediated cerebral damage using an in vitro model of human BBB. In addition, comprehensive analyses of the relationship between PKC-α isoform and RhoA/ pMLC2 pathway were performed to unravel new therapeutic targets that can be utilised to prevent or minimise cerebral barrier damage in hyperglycaemic settings.
Material and Methods
Cell culture
Human BMEC (HBMEC) and astrocytes (HA) were purchased from TCS CellWorks, UK and cultured up to passage 6 in their respective specialised medium in humidified atmosphere under normal conditions (70% N2, 25% O2 and 5% CO2 at 37°C) before exposure to normoglycaemia (5.5 mM D-glucose) or hyperglycaemia (25 mM D-glucose) for 72 hours. The significance of changes in PKC-α activity to HG-mediated endothelial-barrier damage was assessed using a specific inhibitor, Ro-32-0432 (1-5 μM). In a subset of experiments, HBMEC were initially cultured in hyperglycaemic conditions for 72 hours before a further 72 hours culture in normoglycaemic conditions to assess the specific effects of glucose normalisation (glycaemic control). Cells were also cultured in equimolar concentrations of D-mannitol (25 mM) to assess if the changes observed in enzyme activities and BBB integrity were due to hyperosmolality rather than HG per se.
In vitro model of human BBB
HA were seeded on the outside of polyester membrane (0.4 μm pore size) transwell inserts (Corning Costar, High Wycombe, UK) directed upside down in the 6-well tissue culture plates. Following the overnight adherence of HA to the membranes, the inserts were inverted the correct way in the 12-well tissue culture plates and HBMEC were seeded onto the inner side of the polyester membrane. The cells were cultured to confluence before exposing co-cultures to the experimental conditions.
BBB experiments
The integrity and function of human BBB were assessed by measurements of transendothelial electrical resistance (TEER) and the flux of Evan’s blue-labelled albumin (EBA), a high molecular weight permeability marker (67 kDa), across the cocultures as previously described [17].
Immunoblotting
To evaluate the fold differences in protein levels, immunoblottings were performed [15] using the specific antibodies raised against total RhoA, total MLC2, pMLC2-Ser19, pMLC2-Thr18-Ser19, PKC-α, PKC-β (Santa Cruz Biotech) and β-actin (Sigma-Aldrich, UK).
Immunofluorescence
HBMEC cultured on 8-chamber tissue slides (Lab-Tek) were fixed and permeabilised with 4% paraformaldehyde/PBS and 0.1% Triton X-100/PBS, respectively for 20 min each before visualising actin cytoskeleton via Rhodamine-labelled phalloidin dye (5 U/ mL, Invitrogen, UK). The cells were stained with 4,6-diamidino- 2-phenylindole to visualise the nuclei and viewed under 40X magnification on a Zeiss Axio Observer fluorescence microscope (Germany).
Rho activity assay
The RhoA activity was measured using a “pull-down” assay kit (Millipore, UK). Briefly, about 150 μg of HBMEC lysates were incubated with Rhotekin Rho-binding peptide immobilized on agarose beads (10 μg) prior to detection of activated guanosine triphosphate-RhoA bound to Rhotekin by immunoblotting.
PKC activity assay
Total PKC activity was determined in HBMEC lysates as previously described using the non-radioactive PKC activity assay kit (Promega, UK) which utilises a brightly coloured PKC-specific peptide substrate, PepTag C1 [17].
Transient knockdown
To knockdown PKC-α protein expression, semi-confluent HBMEC were transfected for 24 hours with DharmaFECT siRNA transfection reagent-4 containing 50 nM of either ON-TARGET plus non-targeting pool of small interfering RNA (siRNA) or ON-TARGET plus SMART pool human siRNA against PKC-α (GE Dharmacon). Following first round of transfection, the cells were allowed to recover in the fresh medium for 6 hours before second round of transfection with 50 nM of aforementioned siRNA molecules. The cells were homogenised after 72 hours to assess the downregulation of protein expression by immunoblotting.
Assessment of cell viability
A fraction of cells exposed to different treatments were incubated with 0.1% trypan blue for 5 minutes and the percentages of cell viability were calculated by counting 100 cells on a haemocytometer under light microscope.
Statistical Analysis
Statistical analyses were performed using IBM SPSS statistics 21.0 software package. The mean values were compared by Student’s two-tailed t-test or one-way ANOVA, where appropriate, followed by Dunnet’s post-hoc analysis. P<0.05 was considered significant.
Results
Inhibition of PKC-α protects BBB integrity and function under hyperglycaemic conditions
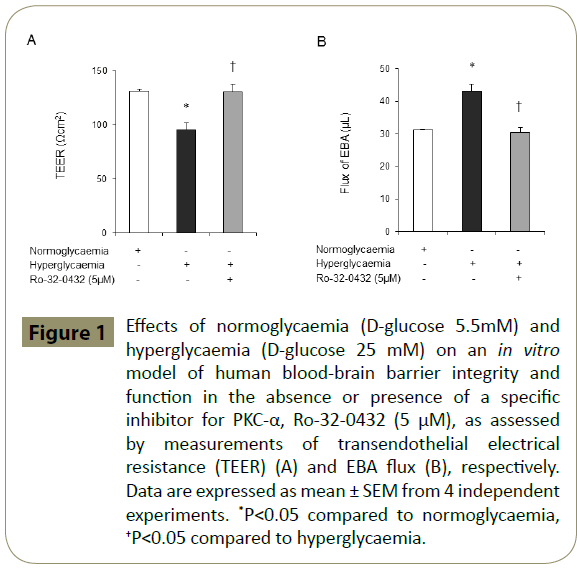
Using an in vitro model of human BBB established by co-culture of HBMEC and HA, we have recently shown that inhibition of total PKC with Bis-I negates the deleterious effects of hyperglycaemia on BBB integrity [14]. The present study shows that specific attenuation of PKC-α activity by Ro-32-0432 also normalises the disruptive effects of hyperglycaemia on cerebral barrier structure and function (Figure 1A-B).
Figure 1: Effects of normoglycaemia (D-glucose 5.5mM) and hyperglycaemia (D-glucose 25 mM) on an in vitro model of human blood-brain barrier integrity and function in the absence or presence of a specific inhibitor for PKC-α, Ro-32-0432 (5 μM), as assessed by measurements of transendothelial electrical resistance (TEER) (A) and EBA flux (B), respectively. Data are expressed as mean ± SEM from 4 independent experiments. *P<0.05 compared to normoglycaemia, †P<0.05 compared to hyperglycaemia.
PKC-α modulates the activation of RhoA signalling in hyperglycaemic settings
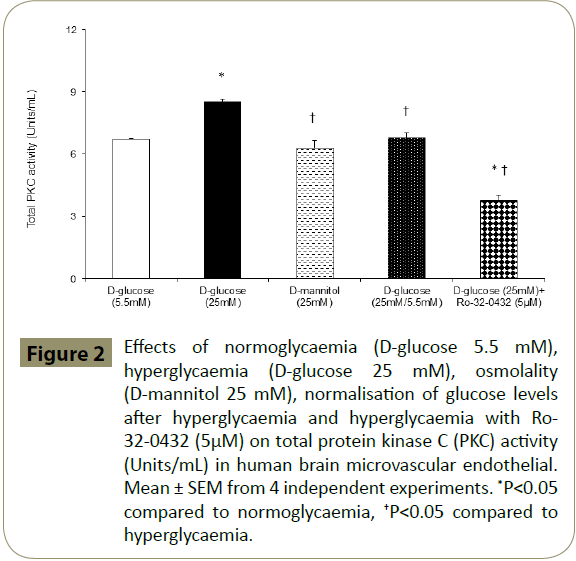
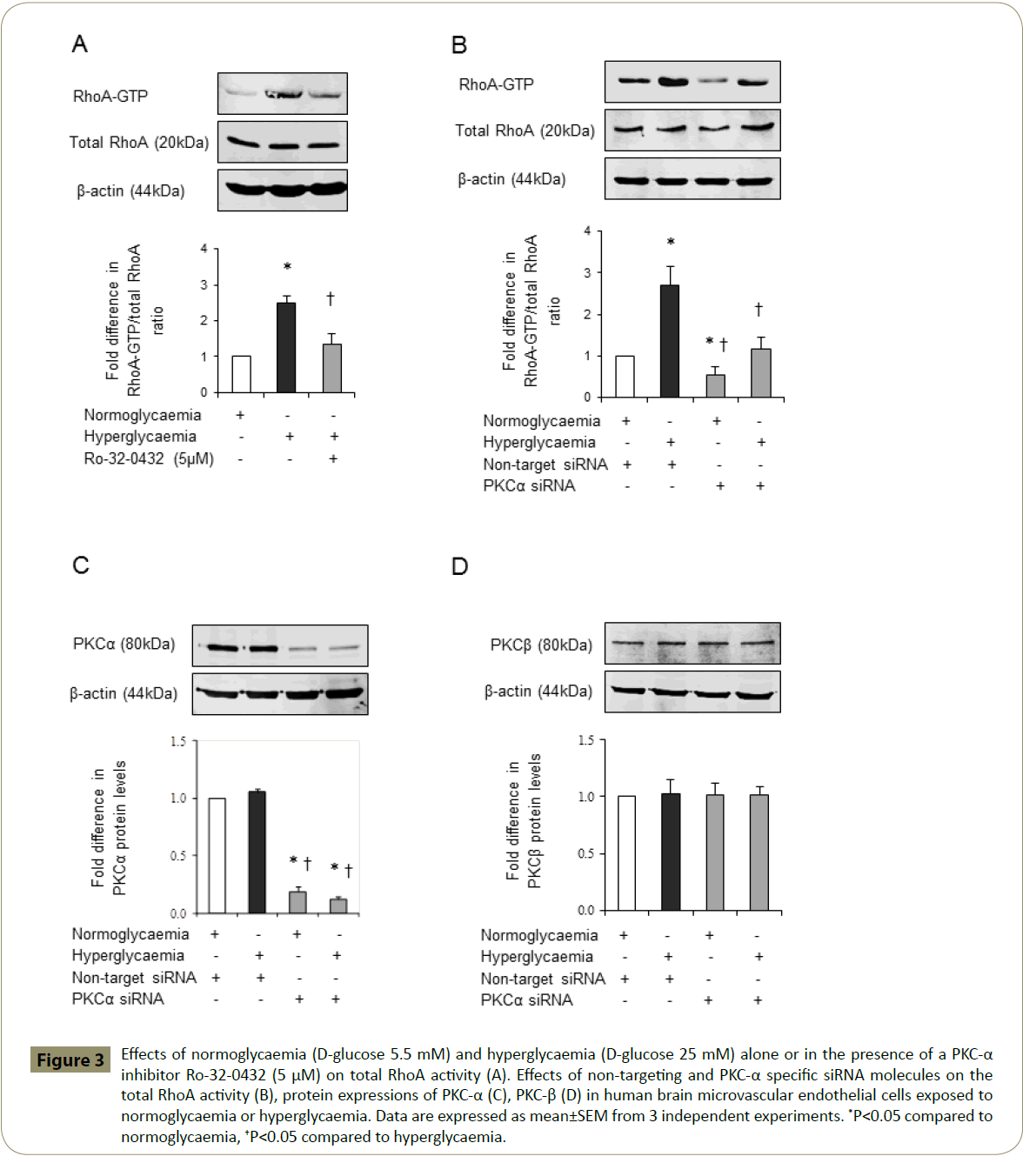
Since overactivities of PKC and RhoA have previously been implicated in cerebral barrier dysfunction [15,17-19] and Ro- 32-0432 effectively maintains barrier integrity and function in hyperglycaemic settings, it is possible that inhibition of PKC-α may protect BBB by concomitantly regulating the activities of total PKC and RhoA. Indeed, inhibition of PKC-α reduces the total PKC activity in HBMEC below the levels seen in cells exposed to equal periods of hyperglycaemia, normoglycaemia or hyperglycaemia followed by normoglycaemia (Figure 2). Moreover, inhibition of PKC-α normalised the hyperglycaemia-mediated increases observed in RhoA-GTP protein levels, a measure of RhoA activity without affecting that of total RhoA (Figure 3A). In support of this, attenuation of PKC-α gene expression by a specific siRNA appeared to diminish RhoA-GTP protein levels without affecting total RhoA levels in HBMEC exposed to normoglycaemia and hyperglycaemia. In these experiments, suppression of the basal RhoA activity in normoglycaemic cells by PKC-α siRNA confirmed the efficacy of this experimental procedure (Figure 3B). Moreover, the inefficacy of PKC-α siRNA to affect PKC-β protein expression confirmed the specificity of the siRNAs employed for PKC-α (Figure 3C,D).
Figure 2: Effects of normoglycaemia (D-glucose 5.5 mM), hyperglycaemia (D-glucose 25 mM), osmolality (D-mannitol 25 mM), normalisation of glucose levels after hyperglycaemia and hyperglycaemia with Ro- 32-0432 (5μM) on total protein kinase C (PKC) activity (Units/mL) in human brain microvascular endothelial. Mean ± SEM from 4 independent experiments. *P<0.05 compared to normoglycaemia, †P<0.05 compared to hyperglycaemia.
Figure 3: Effects of normoglycaemia (D-glucose 5.5 mM) and hyperglycaemia (D-glucose 25 mM) alone or in the presence of a PKC-α inhibitor Ro-32-0432 (5 μM) on total RhoA activity (A). Effects of non-targeting and PKC-α specific siRNA molecules on the total RhoA activity (B), protein expressions of PKC-α (C), PKC-β (D) in human brain microvascular endothelial cells exposed to normoglycaemia or hyperglycaemia. Data are expressed as mean±SEM from 3 independent experiments. *P<0.05 compared to normoglycaemia, †P<0.05 compared to hyperglycaemia.
PKC-α mediates hyperglycaemia-evoked cytoskeletal remodelling
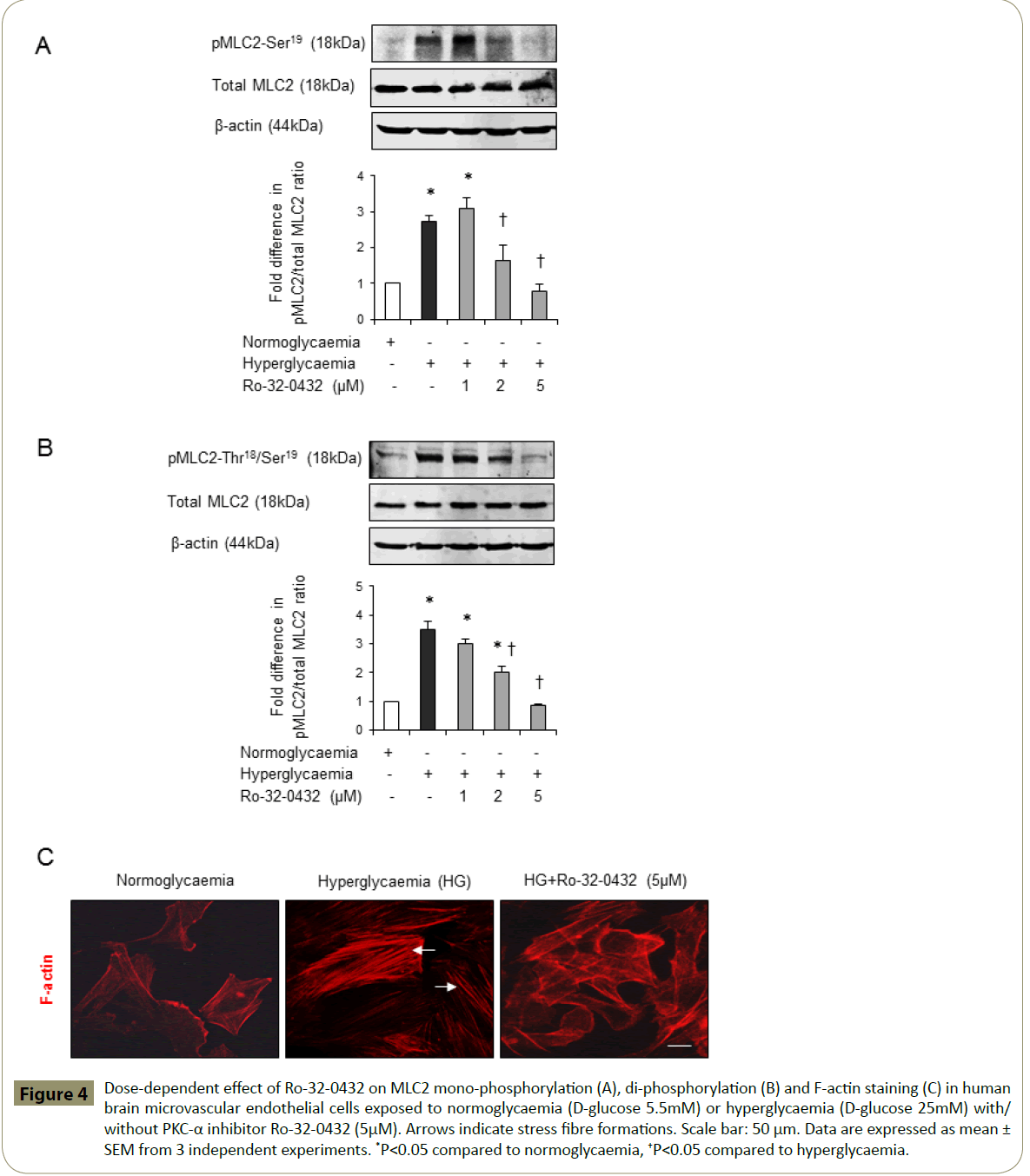
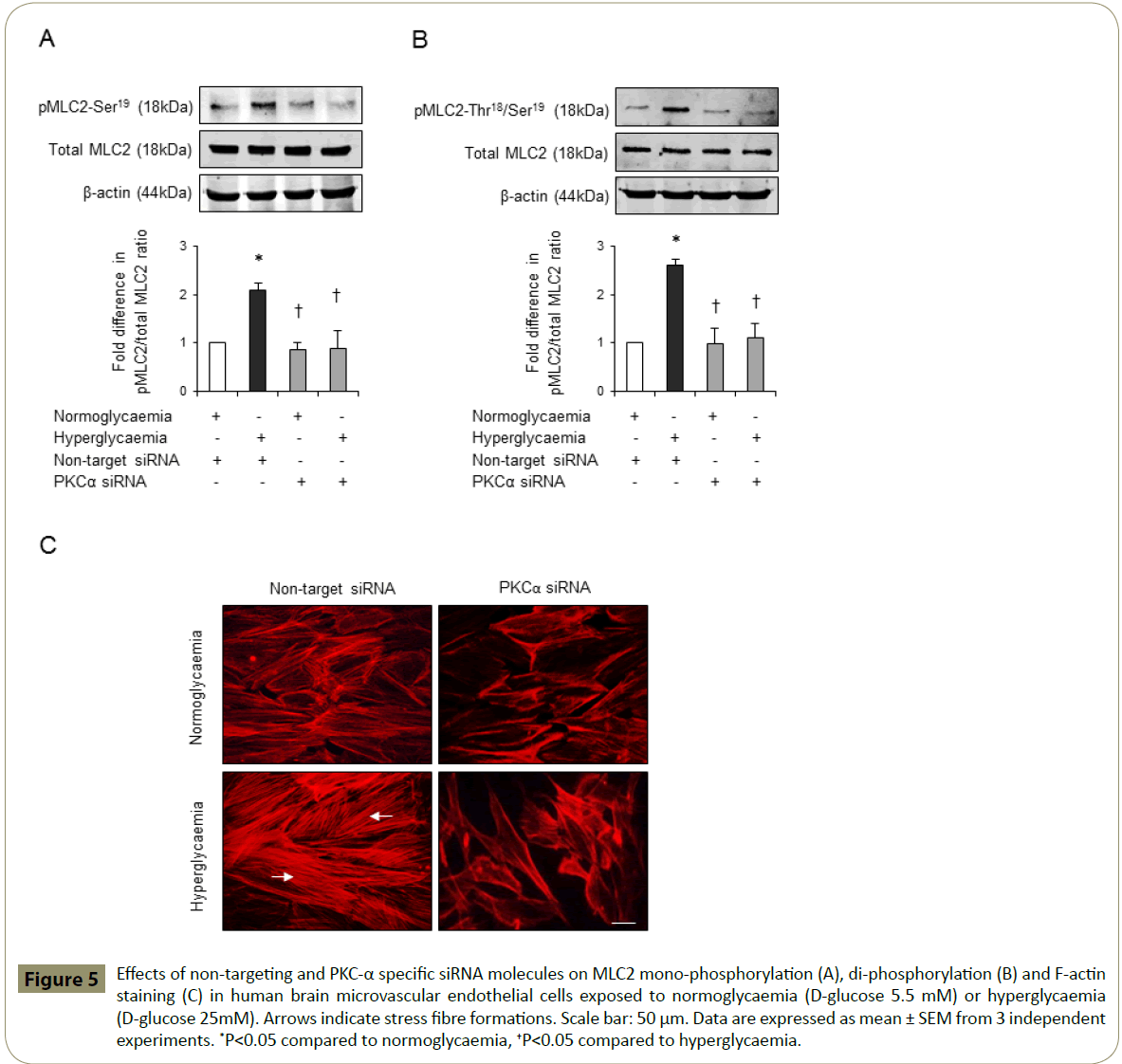
Cytoskeletal reorganisation induced by activation of RhoA has previously been coupled to enhanced cerebral barrier permeability [15,17,19]. To evaluate whether barrier-protective effects of PKC-α inhibition may in part be attributed to its modulatory effects on endothelial cell cytoskeletal proteins MLC2 and F-actin, this study examined the effects of hyperglycaemia on MLC2 mono- and di-phosphorylation levels and F-actin cellular localisation in the absence or presence of Ro-32-0432. While hyperglycaemia alone evoked a dramatic increase in the protein levels of pMLC2-Ser19 and pMLC2-Thr18/Ser19, co-treatment with Ro-32-0432 suppressed both phosphorylation rates in a dose-dependent manner without altering the total MLC2 levels (Figure 4A-B). In addition to this, hyperglycaemia promoted the formation of stress fibres traversing the cells. However, co-treatments with Ro-32-0432 eradicated stress fibres and restored cortical actin localisation similar to those seen in normoglycaemic cells (Figure 4C). Silencing of PKC-α expression in normoglycaemic and hyperglycaemic HBMEC via siRNA technology fully corroborated these results (Figure 5A-C).
Figure 4: Dose-dependent effect of Ro-32-0432 on MLC2 mono-phosphorylation (A), di-phosphorylation (B) and F-actin staining (C) in human brain microvascular endothelial cells exposed to normoglycaemia (D-glucose 5.5mM) or hyperglycaemia (D-glucose 25mM) with/ without PKC-α inhibitor Ro-32-0432 (5μM). Arrows indicate stress fibre formations. Scale bar: 50 μm. Data are expressed as mean ± SEM from 3 independent experiments. *P<0.05 compared to normoglycaemia, †P<0.05 compared to hyperglycaemia.
Figure 5: Effects of non-targeting and PKC-α specific siRNA molecules on MLC2 mono-phosphorylation (A), di-phosphorylation (B) and F-actin staining (C) in human brain microvascular endothelial cells exposed to normoglycaemia (D-glucose 5.5 mM) or hyperglycaemia (D-glucose 25mM). Arrows indicate stress fibre formations. Scale bar: 50 μm. Data are expressed as mean ± SEM from 3 independent experiments. *P<0.05 compared to normoglycaemia, †P<0.05 compared to hyperglycaemia.
Effects of glucose normalisation and osmolality on enzyme activities and BBB integrity
Hyperglycaemia-induced increases observed in total RhoA activity and impairments in BBB integrity and function were independent of a rise in osmolality as ascertained by application of equal concentrations of D-mannitol (25 mM) and were completely neutralised by normalisation of glucose levels (Table 1).
| Trearment groups | Total RhoA activity (AU) | TEER(Ωcm2) | EBA flux (ΜL) |
|---|---|---|---|
| D-glucose (5.5mM) | 1.0 ±0.00 | 125.7±10 | 30.3±6.5 |
| D-glucose (25mM) | 3.0±0.40* | 82.6±5.5* | 47±5.5* |
| D-glucose (25mM) | 1.3±0.20† | 128±3.5† | 28.5±3.9† |
| D-glucose (25mM/5.5mM) | 1.5±0.45† | 115.5±8.2† | 31.2±5.5† |
Mean±SEM from 4 independent experiments *P<0.05 compared to normoglyaemia,†P<0.05 compared to hyperglycaemia. BBB=Blood-brain barrier, PKC= protien kinase C, AU=Arbitatry unit, TEER=Transendothelial electrical resistance,EBA=Evan's blue-labelled albumin.
Table 1: Hyperglycaemia-induced increases observed in total RhoA activity and impairments in BBB integrity and function were independent of a rise in osmolality as ascertained by application of equal concentrations of D-mannitol (25 mM) and were completely neutralised by normalisation of glucose levels.
Discussion
hyperglycaemia stemming from an imbalance in glucose metabolism adversely affects vascular homeostasis and exacerbates the intensity of vasculopathies, notably endothelial dysfunction commonly encountered in patients with history of myocardial infarction and stroke. Although endothelial dysfunction in cerebral vasculature is intimately associated with the initiation and/or progression of BBB failure, the molecular mechanism(s) involved remains largely unknown. Using an in vitro model of human BBB composed of HBMEC and astrocytes, the current study reveals PKC-α as an important component in hyperglycaemia-mediated BBB dysfunction. Activation of PKC-α has been shown to enhance the levels of small GTP-binding protein, RhoA which in turn successively modulates phosphorylation and localisation of cytoskeletal proteins, namely MLC2 and actin microfilaments and as a consequence promote cytoskeletal reorganisation and BBB leakage. Inhibition of PKC-α activity under hyperglycaemic conditions by Ro-32-0432 or PKC-α siRNA appear to alleviate cytoskeletal remodelling and improve endothelial barrier integrity and function by normalising the RhoA activity. Repetition of similar experiments with equimolar concentration of D-mannitol (25 mM) overtly indicates that these changes were independent of an increase in osmolality, in keeping with the findings of our previous studies [14,17]. In addition, normalisation of glucose levels after a period of hyperglycaemic insult (72 hours) appeared to mitigate the effects of hyperglycaemia on total PKC and RhoA activities and on cerebral-barrier integrity, indicating the importance of effective glycaemic control. Indeed, while poor glycaemic control in patients with type 2 diabetes has been positively correlated with an elevated plasma level of vascular endothelial growth factor and development of diabetic retinopathy, its effective regulation, confirmed by decreases in gylcated haemoglobin (HbA1c) levels, appeared to prevent the progression of diabetic retinopathy [20]. Similarly, efficacious management of hyperglycaemia in streptozotocindiabetes rats has been shown to enhance the expression of an endoprotective transcription factor, Kruppel-like factor 2 and attenuate proteinuria, glomerular hypertrophy and endothelial injury, hallmarks of diabetic nephropathy [21].
Considering that PKC regulates a wide range of activities individually or collectively associated with endothelial (dys) function like excessive production of reactive oxygen species, cytokines, growth factors and the activation of matrix metalloproteinases [5], it was of no surprise that treatments with Ro-32-0432 (1-5 μM), an inhibitor of PKC-α restored BBB integrity and function under hyperglycaemic conditions in a dosedependent manner. The concentrations of Ro-32-0432 employed in this study were in the previously reported working range (1-10 μM) and did not markedly affect HBMEC viability similar to human lung epithelial cells, platelets and monocytes [22-24]. As 5 μM of Ro-32-0432 diminished RhoA-GTP activity to the levels obtained by silencing of PKC-α gene via its siRNA, this dose was used during the rest of the study.
Exposure of HBMEC to hyperglycaemia potentiated the activity of total PKC which was reduced by co-treatment with Ro-32-0432 below the levels observed in normoglycaemic cells, implying effective neutralisation of both basal and induced levels of PKC-α activity with this particular inhibitor. These results suggest that activation of PKC-α significantly contributes to the enhanced overall PKC activity and ensuing vascular pathologies in hyperglycaemic conditions. Indeed, activation of PKC-α in diabetic rats has been shown to enhance the NADPH oxidasemediated superoxide production in renal tissues and elevate the renal, serum, and urinary vascular endothelial growth factor concentrations leading to diabetic nephropathy [25]. Similarly, activation of PKC-α has been coupled to increased incidence of heart failure in mice where its effective inhibition improved ventricular performance and reduced fibrosis and pulmonary oedema [26].
Similar to total PKC, exposure of HBMEC to hyperglycaemia also resulted in an increase in RhoA activity. Like PKC, small GTPase RhoA is also implicated in various processes that can radically affect the integrity and permeability of the BBB. Once activated, RhoA has been shown to bind to its effector protein Rho-kinase and promote stress fibre formation and MLC2 phosphorylation which in turn increase paracellular flux by destabilising cell junctions thereby suggesting the notion that inhibition of RhoA may be of paramount importance in attaining normal microvascular function and negating barrier permeability [15,27,28]. Indeed, inhibition of Rho-kinase activity by Y-27632 has been shown to completely abolish these effects in different conditions known to affect BBB integrity including hyperglycaemia and oxygen-glucose deprivation alone or followed by reperfusion [15,17]. In accordance with these findings, in the current study hyperglycaemia has been shown to trigger MLC2 phosphorylation and cytoskeletal reorganisation characterised by appearance of thick actin stress fibres traversing the cells. Interestingly, inhibition of PKC-α activity by Ro-32-0432 in HBMEC mimicked the endothelial-barrier protective effects of Y-27632 by normalising the RhoA activity, mono- and di-phosphorylation of MLC2, reducing stress fibre formations and restoring the F-actin localisation to the cellular cortex [17]. These results indicate that PKC-α can effectively modulate the RhoA/Rho-kinase signalling pathway. The importance of this pathway has been exemplified in a recent study with retinal pigment epithelial cells where PKC-α overactivity is associated with enhanced cell migration triggered by the formation of stress fibres and actin cytoskeletal remodelling contributing to the development of proliferative eye diseases [29]. Furthermore, in vivo studies with mice and in vitro studies with human pulmonary artery endothelial cells have also shown that activation of PKC-α with microtubule-disrupting agents like 2-methoxyestradiol increase endothelial-barrier permeability by phosphorylating ezrin, radixin and moesin proteins, known to activate the Rho signalling and promote de novo assembly of F-actin [30-32].
In addition to its effects on cellular architecture, activation of RhoA also affects localisation of occludin and zonula occludens-1, tight junction proteins that prevent paracellular leakage [17]. Besides, RhoA activation potentiates the activities of matrix metalloproteinases which digest extracellular matrix components and thus enhance endothelial-barrier permeability [33]. Since Ro-32-0432 may also interfere with the activities of PKC-β [23,34], the specific relevance of PKC-α to RhoA activity, MLC2 phosphorylation and cytoskeletal remodelling in hyperglycaemic settings was investigated in HBMEC after silencing its gene expression by siRNAs which had no effect on PKC-α protein expression thereby confirming specificity. The results generated were similar to those obtained with Ro-32-0432, confirming the regulatory role of PKC-α in RhoA activation and supporting the previous studies showing PKC-α-mediated activation of RhoA at cell membrane by either regulating its dissociation from Rho guanine dissociation inhibitor 1-RhoA complex to promote its cytoplasmic trafficking as seen in pancreatic acini or by phosphorylating p115Rho guanine exchange factor to facilitate the exchange of GDP with GTP as reported in mouse BMEC [35]. Along with promoting cytoskeletal remodelling, enhanced PKC-α activity may also compromise endothelial barrier integrity by inducing apoptosis, matrix metalloproteinase activity or oxidative stress through modulating the excessive synthesis of reactive oxygen species, in particular, superoxide anion by prooxidant enzyme NADPH oxidase and/or suppressing nitric oxide availability, a key regulator of vascular homeostasis by uncoupling endothelial nitric oxide synthase (eNOS) [36,37]. Hence, selective inhibition of conventional PKC isoforms in type 2 diabetic rats by Ro-32-0432 and Go-6976 have been shown to mitigate oxidative stress in conduit arteries by both restoring eNOS function and attenuating NADPH oxidase hyperactivity [38,39]. Furthermore, siRNA-mediated inhibition of PKC-α in HBMEC neutralised the barrier-disruptive effects of oxygen-glucose deprivation and TNF-α, an inflammatory cytokine by attenuating oxidative stress, apoptosis and the activity of basement membrane-degrading enzymes [40].
It is noteworthy here that both normalisation of glucose levels and targeted silencing the activities of other conventional isoforms, namely PKC-β, PKC-βI or PKC-βII have previously been shown to improve in vitro BBB integrity and function in hyperglycaemic settings by negating the stimulatory effects of hyperglycaemia on endothelial cell apoptosis, cytoskeletal remodelling, oxidative stress, NADPH oxidase and MMPs activities and RhoA/Rho-kinase/ MLC2 expression, activity and localisation. Inhibition of PKC-α in these studies has also been shown to attenuate apoptosis and NADPH oxidase activation in hyperglycaemic HBMEC [17,18,41].
Taken together with the current data, our results collectively indicate that activation of conventional PKC isoform i.e. PKC-α may augment the extent and severity of BBB damage in neurodegenerative conditions such as ischaemic stroke accompanied by uncontrolled diabetes or stress hyperglycaemia by concomitantly inducing various interrelated molecular mechanisms and that pharmacological inhibition of this isoform can both successfully maintain barrier function and diminish the incidence of secondary complications [42]. Bearing in mind the results of our previous studies and the necessity to develop efficacious therapeutic regimens that can be used as alternatives to the currently available sole medical therapy with recombinant tissue plasminogen activator after diabetic stroke, it is important to extend the present study to a translational model in order to investigate whether simultaneous suppression of multiple conventional PKC isoforms may exert better protection on cerebral barrier as compared to inhibition of a single isoform.
Acknowledgement
The authors would like to acknowledge The University of Nottingham, UK for conducting the PhD programme in Clinical Neurosciences, which led to the preparation of this manuscript.
Funding
The authors like to acknowledge The University of Nottingham, UK for funding the study in form of PhD studentship.
References
- Baird TA, Parsons MW, Barber PA, Butcher KS, Desmond PM, et al. (2002) The influence of diabetes mellitus and hyperglycaemia on stroke incidence and outcome. J Clin Neurosci 9: 618-626.
- Hacke W, Schwab S, Horn M, Spranger M, DeGeorgia M, et al. (1996) 'Malignant' middle cerebral artery territory infarction-Clinical course and prognostic signs. Arch Neurol 53: 309-315.
- Kiers L, Davis SM, Larkins R, Hopper J, Tress B, et al. (1992) Stroke topography and outcome in relation to hyperglycaemia and diabetes. J Neurol Neurosurg Psychiatry 55: 263-270.
- Bayraktutan U (2002) Free radicals, diabetes and endothelial dysfunction. Diabetes Obes Metab 4: 224-238.
- Geraldes P, King GL (2010) Activation of Protein Kinase C isoforms and its impact on diabetic complications. Circ Res 106: 1319-1331.
- Inoguchi T, Battan R, Handler E, Sportsman JR, Heath W, et al. (1992) Preferential elevation of protein kinase C isoform beta-II and diacylglycerol levels in the aorta and heart of diabetic rats-Differential reversibility to glycemic control by islet cell transplantation. Proc Natl Acad Sci U S A 89: 11059-11063.
- Shiba T, Inoguchi T, Sportsman JR, Heath WF, Bursell S, et al. (1993) Correlation of diacylglycerol level and protein kinase C activity in rat retina to retinal circulation. Am J Physiol 265: E783-E793.
- Ishii H, Koya D, King GL (1998) Protein kinase C activation and its role in the development of vascular complications in diabetes mellitus. J Mol Med-Jmm 76:21-31.
- Chen ML, Pothoulakis C, LaMont JT (2002) Protein kinase C signaling regulates ZO-1 translocation and increased paracellular flux of T84 colonocytes exposed to Clostridium difficile toxin A. J Biol Chem 277: 4247-4254.
- Harhaj NS, Felinski EA, Wolpert EB, Sundstrom JM, Gardner TW, et al. (2006) VEGF activation of protein kinase C stimulates occludin phosphorylation and contributes to endothelial permeability. Invest Ophthalmol Vis Sci47: 5106-5115.
- Hink U, Li HG, Mollnau H, Oelze M, Matheis E, et al. (2001) Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88: E14-E22.
- Stamatovic SM, Dimitrijevic OB, Keep RF, Andjelkovic AV (2006) Protein kinase C alpha-RhoA cross-talk in CCL2-induced alterations in brain endothelial permeability. J Biol Chem 281: 8379-88.
- Xu YH, Wang SS, Feng LA, Zhu QA, Xiang P, et al. (2010) Blockade of PKC-beta protects HUVEC from advanced glycation end products induced inflammation. Int Immunopharmacol 10: 1552-1559.
- Allen CL, Bayraktutan U (2009) Antioxidants attenuate hyperglycaemia-mediated brain endothelial cell dysfunction and blood-brain barrier hyperpermeability. Diabetes Obes Metab 11: 480-490.
- Allen C, Srivastava K, Bayraktutan U (2010) Small GTPase RhoA and its effector Rho kinase mediate oxygen glucose deprivation- evoked in vitro cerebral barrier dysfunction. Stroke 41: 2056-2063.
- VanAelst L, D'Souza-Schorey C (1997) Rho GTPases and signaling networks. Genes Dev 11: 2295-2322.
- Srivastava K, Shao B, Bayraktutan U (2013) PKC-beta exacerbates in vitro brain barrier damage in hyperglycemic settings via regulation of RhoA/Rho-kinase/MLC2 pathway. J Cereb Blood Flow Metab 33: 1928-1936.
- Shao B, Bayraktutan U (2013) Hyperglycaemia promotes cerebral barrier dysfunction through activation of protein kinase C-beta. Diabetes Obes Metab 15: 993-999.
- Gibson CL, Srivastava K, Sprigg N, Bath PMW, Bayraktutan U (2014) Inhibition of Rho kinase protects cerebral barrier from ischaemia-evoked injury through modulations of endothelial cell oxidtive stress and tight junctions. J Neurochem 129: 816-826.
- Zehetner C, Kirchmair R, Kralinger M, Kieselbach G (2013) Correlation of vascular endothelial growth factor plasma levels and glycemic control in patients with diabetic retinopathy. Acta Ophthalmol 91: e470-e473.
- Zhong F, Chen HB, Wei CG, Zhang W, Li Z, et al. (2015) Reduced Kruppel-like factor 2 expression may aggravate the endothelial injury of diabetic nephropathy. Kidney Int 87: 382-395.
- Lin CH, Cheng HW, Hsu MJ, Chen MC, Lin CC, et al. (2006) c-Src mediates thrombin-induced NF-kappa B activation and IL-8/CXCL8 expression in lung epithelial cells. J Immunol 177: 3427-3438.
- de la Rosa CM, Radziwon-Balicka A, El-Sikhry H, Seubert J, Ruvolo PP, et al. (2013) Pharmacologic Protein Kinase C alpha inhibition uncouples human platelet-stimulated angiogenesis from collagen-induced aggregation. J Pharmacol Exp Ther 345: 15-24.
- Chang MY, Huang DY, Ho FM, Huang KC, Lin WW (2012) PKC-Dependent human monocyte adhesion requires AMPK and Syk activation. PLoS One 7(7).
- Thallas-Bonke V, Thorpe SR, Coughlan MT, Fukami K, Yap FY, et al. (2008) Inhibition of NADPH oxidase prevents advanced glycation end product-mediated damage in diabetic nephropathy through a protein kinase C-alpha-dependent pathway. Diabetes 57: 460-469.
- Liu QH, Chen XW, MacDonnell SM, Kranias EG, Lorenz JN, et al. (2009) Protein Kinase C alpha, but not PKC beta or PKC gamma, regulates contractility and heart failure susceptibility implications for ruboxistaurin as a novel therapeutic approach. Circ Res 105: 194-200.
- Asano T, Suzuki T, Tsuchiya M, Satoh S, Ikegaki I, et al. (1989) Vasodilator actions of HA1077 in vitro and in vivo putatively mediated by the inhibition of protein-kinase. Br J Pharmacol 98: 1091-1100.
- Sahai E, Marshall CJ (2002) Rho-GTPases and cancer. Nat Rev Cancer 2: 133-142.
- Ruiz-Loredo AY, Lopez E, Lopez-Colome AM (2012) Thrombin stimulates stress fiber assembly in RPE cells by PKC/CPI-17-mediated MLCP inactivation. Exp Eye Res 96: 13-23.
- Bogatcheva NV, Zemskova MA, Gorshkov BA, Kim KM, Daglis GA, et al. (2011) Ezrin, radixin, and moesin are phosphorylated in response to 2-methoxyestradiol and modulate endothelial hyperpermeability. Am J Respir Cell Mol Biol 45: 1185-1194.
- Defacque H, Egeberg M, Habermann A, Diakonova M, Roy C, et al. (2000) Involvement of ezrin/moesin in de novo actin assembly on phagosomal membranes. EMBO J 19: 199-212.
- Takahashi K, Sasaki T, Mammoto A, Takaishi K, Kameyama T, et al. (1997) Direct interaction of the Rho GDP dissociation inhibitor with ezrin/radixin/moesin initiates the activation of the Rho small G protein. J Biol Chem 272: 23371-23375.
- Xu RF, Feng XY, Xie X, Zhang J, Wu DC, et al. (2012) HIV-1 Tat protein increases the permeability of brain endothelial cells by both inhibiting occludin expression and cleaving occludin via matrix metalloproteinase-9. Brain Res 1436: 13-19.
- Li LW, Lorenzo PS, Bogi K, Blumberg PM, Yuspa SH (1999) Protein kinase C delta targets mitochondria, alters mitochondrial membrane potential, and induces apoptosis in normal and neoplastic keratinocytes when overexpressed by an adenoviral vector. Mol Cell Biol 19: 8547-8558.
- Peng J, He F, Zhang CL, Deng XL, Yin F (2011) Protein kinase C-alpha signals P115RhoGEF phosphorylation and RhoA activation in TNF-alpha-induced mouse brain microvascular endothelial cell barrier dysfunction. J Neuroinflammation 8: 1-10.
- Chen F, Kumar S, Yu YF, Aggarwal S, Gross C, et al. (2014) PKC-dependent phosphorylation of eNOS at T495 regulates eNOS coupling and endothelial barrier function in response to G(+) -toxins. PLoS One 9: e99823.
- Rakkar K, Bayraktutan U (2016) Increases in intracellular calcium perturb blood-brain barrier via protein kinase C-alpha and apoptosis. Biochim Biophys Acta 1862: 56-71.
- Chen F, Yu Y, Haigh S, Johnson J, Lucas R, et al. (2014) Regulation of NADPH oxidase 5 by Protein Kinase C isoforms. PLoS One 9: e88405.
- Kikuchi C, Kajikuri J, Hori E, Nagami C, Matsunaga T, et al. (2014) Aortic superoxide production at the early hyperglycemic stage in a rat type 2 diabetes model and the effects of pravastatin. Biol Pharm Bull 37: 996-1002.
- Abdullah Z, Bayraktutan U (2016) Suppression of PKC-α attenuates TNF-α-evoked cerebral barrier breakdown via regulations of MMP-2 and plasminogen-plasmin system. Biochim Biophys Acta 1862: 1354-1366.
- Shao B, Bayraktutan U (2014) Hyperglycaemia promotes human brain microvascular endothelial cell apoptosis via induction of protein kinase C-betaI and prooxidant enzyme NADPH oxidase. Redox Biol 2: 694-701.
- Hempel A, Maasch C, Heintze U, Lindschau C, Dietz R, et al. (1997) High glucose concentrations increase endothelial cell permeability via activation of protein kinase C alpha. Circ Res 81: 363-371.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences