Formulation and Optimization of Propranolol Bilayer Tablets: A Potential Approach for Effective Management of Hypertension
Hemant Mourya*, Navneet Garud, Ramakant Joshi, Wasim Akram and Nitin Singh
Department of Pharmaceutical Sciences, Jiwaji University, Gwalior, Madhya pradesh, India
- *Corresponding Author:
- Hemant Mourya
Department of Pharmaceutical Sciences,
Jiwaji University, Gwalior, Madya pradesh,
India,
E-mail: Hemant35143@gmail.com
Received date: August 01, 2022, Manuscript No. IPSRT -22-11045; Editor Assigned date: August 03, 2022, PreQC No. IPSRT -22-11045 (PQ); Reviewed date: August 16, 2022, QC No. IPSR T-22-11045; Revised date: August 23, 2022, Manuscript No. IPSRT -22-11045 (R); Published date: August 29, 2022, DOI: 10.36648/2574-285x.6.4.146
Citation: Mourya H, Garud N, Joshi R, Akram W, Singh N (2022) Formulation and Optimization of Propranolol Bilayer Tablets: A Potential Approach for Effective Management of Hypertension. Stroke Res Ther Vol.6 No.4: 146.
Abstract
The purpose of this investigation is to formulate and evaluate the antihypertensive drug propranolol hydrochloride sustained-release bilayer tablets. In this formulation, one layer provides a loading dose through the immediate release of the drug, and the other layer provides a maintenance dose for up to 12 hours through controlled release. Different quantities of polymers such as Kyron T-314, Hydroxypropyl methylcellulose (HPMC-K4M), and ethyl cellulose were used to make bi-layer tablets by direct compression. The compatibility study of pharmaceutical excipients was conducted through FTIR studies, and no interaction was found. The pre-compression parameter for the angle of repose, bulk density, tapped density, and compressibility index was assessed on the produced granules, and the findings were good. The tablets were evaluated for the post-compression parameters for thickness, hardness, friability, and in-vitro release studies. In-vitro dissolution study was approved out for 12 hours using USP dissolution apparatus I using phosphate buffer of pH 1.2 and 6.8 as dissolution medium. HPMC-K4M and ethylcellulose were used in combination in all formulations but optimized formulation PHT4 showed a higher rate of drug release up to 12 hours as compared to the other formulation and optimized formulations PHT4 follows the Higuchi model with non-fickian diffusion based on regression coefficient of the kinetics data of cumulative drug release from the dosage form.
Keywords
Propranolol hydrochloride; Sustained release bilayer tablet; Hydroxypropyl methylcellulose; Ethyl cellulose.
Introduction
For decades, oral drug delivery has been recognized as the most widely used route of administration in the exploration of systemic drug delivery [1]. When administered in the traditional dosage form, many orally delivered medications have low bioavailability. i.e. the degree and level of absorption of the medications are less than desirable. Absorption of some medications can be as low as 30% or less of the orally managed dosage. Furthermore, poorly absorbed drugs have a lot of variation in bioavailability within and within-subjects. This dilemma could be solved by using a revamped release drug delivery system with a longer stomach residence period [2]. Furthermore, conventional dosage forms cause a wide variety of drug concentration fluctuations in the bloodstream and tissues, resulting in decreased or lost drug potency or a rise in the rate of side effects, resulting in unwanted toxicity and inefficiency. Sustained or managed drug delivery systems, on the other hand, will reduce dosing frequency while still increasing drug efficacy by localizing the drug at the site of operation, lowering the dosage needed, and ensuring uniform drug delivery [3]. An Immediate-Release (IR) layer and a Sustained-Release (SR) layer are used in a bilayer tablet, which is a recent idea for the effective production of a sustained release formulation with different features to provide a means of a successful drug delivery system. The immediate-release layer maintains therapeutically efficient plasma drug concentrations for a limited period, while the Sustained Release (SR) layer maintains uniform drug levels for an extended period, reducing dosing cycles and side effects, increasing the safety boundary for highly potent medications, and thereby improving patient compliance. Hypertension is characterized as a systolic pressure greater than 140 mmHg and a diastolic pressure greater than 90 mmHg with a systolic pressure greater than 140 mmHg. It can induce blood flow changes in the back of the eye (retina), irregular heart muscle thickening, kidney dysfunction, brain injury, myocardial infarction (heart attacks), heart failure, artery aneurysms (e.g. aortic aneurysm), and peripheral arterial disease [4]. Propranolol hydrochloride is a nonselective beta-adrenergic blocker that is commonly used to treat asthma, angina pectoris, and a variety of other cardiovascular conditions [5]. Propranolol hydrochloride has poor oral bioavailability (15%-23%). It is a crystalline solid that is stable and soluble in water and ethanol. It has a molecular weight of 295.80 [6]. It's an extremely water-soluble compound with a 3-5 hour cellular half-life, and the normal dosage is 40 mg three times a day. This necessarily requires a high frequency of administration, resulting in oscillations in plasma medication concentrations. To lower the frequency of administration while increasing potency and oral bioavailability, a sustained release dosage type must be developed [7].
Materials and Methods
Propranolol Hydrochloride as a gift sample from Ipca Lab. Ltd. Ratlam (m.p) India. Hydroxypropyl methylcellulose (HPMC-K4M) and Ethyl cellulose from Loba Chemie Pvt. Ltd. (Mumbai), Starch from Hi-Media Laboratories Pvt. Ltd. (Mumbai), Microcrystalline cellulose (MCC) from Hi-Media Laboratories Pvt. Ltd. (Mumbai), Magnesium stearate from Central Drug House Pvt. Ltd. (New Delhi). All other chemicals used were of analytical grade.
Fourier Transform Infrared Spectroscopy (FTIR)
The functional groups in a molecule are identified using FTIR. The medication was scanned at 4 mm/s with a resolution of 2 cm on a wavenumber range of 400 to 4000cm-1 on a KBr disc. Since the drug and polymer are nearby during the preparation of a bilayer pill, they can interfere, resulting in drug instability. Preformulation experiments on drug-polymer interactions are also crucial in choosing the right polymers. The stability of propranolol hydrochloride and selected polymers was determined using FTIR spectroscopy (Perkin Elmer model no. spectrum two serial no. 105627 FT-IR). Separately, the actual medication and the drug with excipients were scanned [8].
Differential Scanning Colorimetry (DSC)
DSC measurements of propranolol hydrochloride were approved out using a thermo tropic transformation testing tool (DSC 60 plus Shimadzu Japan). Empty aluminum pans were used as controls, and samples were carefully put in another aluminum pan. The test was carried out in an inert environment at a rate of 10oC per minute in a temperature range of 30oC to 305oC [9].
Pre-compression parameters
The powder blend was evaluated for pre-compression properties like angle of repose, bulk density, tapped density, Carr’s index, Hausner’s ratio [10].
Preparation of bilayer tablet
The tablets are manufactured by compressing powdered ingredients without changing their physical properties. Direct compression is usually used for crystalline materials with good physical features like flow, compressibility, and so on. Direct compression has several advantages, including time savings, operational safety and cheap cost.
Dose calculation
For sustained drug release up to 12 hours, the immediate dose of drug was calculated from total dose of Propranolol hydrochloride extended release tablet, which was 80mg.According pharmacokinetic data [11]. DT=AD (1+0.693×t/ t1/2) where, DT=Total Dose, AD=Adult Dose, T=Total time period for which sustained release is required, t1/2 = Half-life of drug. Half-life of Propranolol hydrochloride ranges from 3 to 5 hours. For example, i. Propranolol hydrochloride: 80=Dose [1+(0.693×12)/3)], Dose=21.208 mg Propranolol hydrochloride. ii. Propranolol hydrochloride: 80=Dose [1+(0.693×12)/5)], Dose=30.039 mg Propranolol hydrochloride.
According to dose calculation, IR dose of the drug can be taken in an in-between range of 21.208 mg to 30.039 mg for the preparation of bilayer tablets; thus 22 mg of Propranolol hydrochloride was taken in the IR layer and 58 mg of Propranolol hydrochloride was taken in SR layers.
Formulation of the immediate release layer
Formulation of the immediate-release layer has been done as follows. The powder combination was blended for 15 minutes to achieve a uniform distribution of the medicine in the formulation after the composition of the immediate-release layer was weighed properly and added to the blender. The mixture was blended for 2 minutes with talc and magnesium stearate before being stored in a desiccator until needed Table 1.
| S.No. | Ingredients (mg) | Formulation of immediate release layer (mg) |
|---|---|---|
| 1 | Propranolol hydrochloride | 22 |
| 2 | Kyron T-314 | 4 |
| 3 | Starch powder | 10 |
| 4 | Microcrystalline cellulose (MCC) | 59.8 |
| 5 | Talc | 2 |
| 6 | Magnesium stearate | 2 |
| 7 | Color | 0.2 |
| Total weight | 100 | |
Table 1: Formulation of immediate-release layer.
Formulation of the sustained release layer
Formulation of the sustained-release layer has been done as follows. The powder combination was blended for 20 minutes to achieve a uniform distribution of the medicine in the formulation after the composition of the sustained-release layer was correctly weighed and added to the blender Table 2.
| S.No. | Ingredients (Mg) | Formulation of sustained release layer | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PHT1 (mg) | PHT 2 (mg) | PHT 3 (mg) | PHT 4 (mg) | PHT 5 (mg) | PHT 6 (mg) | PHT 7 (mg) | PHT 8 (mg) | ||
| 1 | Propranolol Hydrochloride | 58 | 58 | 58 | 58 | 58 | 58 | 58 | 58 |
| 2 | Hpmc-K4M | 5 | 10 | 15 | 20 | 25 | 30 | 35 | 40 |
| 3 | Ethylcellulose | 40 | 35 | 30 | 25 | 20 | 15 | 10 | 5 |
| 4 | Starch Powder | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| 5 | Microcrystalline Cellulose (MCC) | 69 | 69 | 69 | 69 | 69 | 69 | 69 | 69 |
| 6 | Talc | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 7 | Magnesium Stearate | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| Total Weight | 200 | 200 | 200 | 200 | 200 | 200 | 200 | 200 | |
Table2: Formulation of sustained-release layer. PHT- Propranolol Hydrochloride Tablet.
Compression of bilayer tablet
The Compression of the bilayer tablet was done manually using single rotatory compression machines (CLIT Single Rotatory 16). An accurately weighed amount of sustainedrelease powder mixture was fed manually into the die cavity. Sustained-release was compressed at mild compression force (2-3 kg/cm2). After that, an accurately weighted immediaterelease powder mixture was manually fed into the die on a sustained release layer and compressed using 9mm circular punches. Both the layers were identified based on color since the immediate release layer had pink color and the sustained release layer has a white color Table 3.
| Formulation Code | Angle Of Repose | Bulk Density | Tapped Density | Compressibility Index (%) | Hausner’s Ratio |
|---|---|---|---|---|---|
| PHT 1 | 32.68 ± 0.36 | 0.47 ± 0.04 | 0.554 ± 0.01 | 13.24 ± 0.65 | 1.10 ± 0.06 |
| PHT 2 | 31.38 ± 0.60 | 0.47 ± 0.01 | 0.558 ± 0.03 | 13.56 ± 0.42 | 1.11 ± 0.14 |
| PHT 3 | 33.51 ± 0.54 | 0.48 ± 0.06 | 0.551 ± 0.05 | 11.43 ± 0.64 | 1.11 ± 0.21 |
| PHT 4 | 30.87 ± 0.12 | 0.48 ± 0.05 | 0.559 ± 0.02 | 12.49 ± 0.32 | 1.10 ± 0.07 |
| PHT 5 | 34.71 ± 0.71 | 0.49 ± 0.03 | 0.553 ± 0.01 | 12.23 ± 0.49 | 1.12 ± 0.14 |
| PHT 6 | 32.26 ± 0.18 | 0.49 ± 0.07 | 0.569 ± 0.04 | 13.78 ± 0.42 | 1.13 ± 0.18 |
| PHT 7 | 33.19 ± 0.96 | 0.50 ± 0.02 | 0.555 ± 0.02 | 11.56 ± 0.32 | 1.12 ± 0.07 |
| PHT 8 | 34.56 ± 0.73 | 0.47 ± 0.03 | 0.563 ± 0.04 | 12.98 ± 0.14 | 1.11 ± 0.21 |
Table 3: Precompression parameters of powder blend. Values are expressed as mean ± S D., n=3.
Post compression parameters
Thickness
Using a digital vernier scale, the thickness of 20 pre-weighed tablets from each batch was assessed, and the normal thickness in mm was calculated. The thickness of the pill should be kept within a 3% range of a standard Table 4 [12].
| Formulation Code | Thickness (Mm) | Hardness (Kg/Cm2) | Friability (%) | Weight Variation (Mg) | % Drug Content |
|---|---|---|---|---|---|
| PHT 1 | 4.06 ± 0.32 | 5.21 ± 0.12 | 0.12 ± 0.09 | 299.89 ± 0.29 | 93.25 ± 0.54 |
| PHT 2 | 4.18 ± 0.49 | 4.87 ± 0.35 | 0.19 ± 0.14 | 299.92 ± 0.21 | 94.29 ± 0.36 |
| PHT 3 | 4.59 ± 0.13 | 4.23 ± 0.89 | 0.25 ± 0.12 | 299.90 ± 0.12 | 96.13 ± 0.12 |
| PHT 4 | 4.02 ± 0.46 | 4.12 ± 0.18 | 0.31 ± 0.13 | 300.01 ± 0.26 | 98.06 ± 0.48 |
| PHT 5 | 4.49 ± 0.56 | 4.01 ± 0.65 | 0.26 ± 0.06 | 299.96 ± 0.21 | 97.14 ± 0.32 |
| PHT 6 | 4.32 ± 0.03 | 4.32 ± 0.56 | 0.19 ± 0.12 | 299.94 ± 0.30 | 95.32 ± 0.64 |
| PHT 7 | 4.21 ± 0.09 | 4.17 ± 0.23 | 0.21 ± 0.09 | 299.96 ± 0.91 | 96.14 ± 0.47 |
| PHT 8 | 4.11 ± 0.29 | 4.19 ± 0.12 | 0.07 ± 0.04 | 299.90 ± 0.29 | 95.12 ± 0.17 |
Table 4: Post compression parameter. Values are expressed as mean ± S D., n=3.
Hardness
It was determined by using Monsanto hardness tester and the average pressure of (kg/cm2) applied for crushing the tablet was determined [13].
Friability
It was determined by first weighing 10 tablets and placing them in Roche Friabilator, which was rotated for 100 revolutions at 25 rpm. After dusting, the total remaining mass of the tablets was recorded and the percent friability was calculated [14].
Weight Variation
According to USP 20 tablets were weighed individually which were randomly selected for the determination of weight variation. The mean and standard deviation were determined [15].
Drug content assay
Ten pills were coarsely pulverized, and 100 mg of Propranolol hydrochloride was properly weighed and placed in a 100 ml volumetric flask, followed by 70 ml of buffer pH 1.2 (0.01N HCl). For ten minutes, the flask was shaken. Finally, using the same buffer solution, the volume was brought up to par. The resulting solution was filtered complete Whatman filter paper (No.41), and 1 ml of the filtrate was diluted to 100 ml with the same buffer solution and analyzed for Propranolol hydrochloride content at 290 nm using a double beam UV/Visible spectrophotometer (Shimadzu 1800, Japan) and 0.01N Hcl as a blank Table 5 [16].
| Time (Hrs) | Cumulative % drug release | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | |
| 1 | 1.2 | 04.89 ± 0.51 | 04.46 ± 0.14 | 06.90 ± 0.35 | 07.47 ± 0.49 | 06.47 ± 0.36 | 04.23 ± 0.29 | 03.29 ± 0.32 | 03.05 ± 0.71 |
| 2 | 1.2 | 07.14 ± 0.12 | 06.39 ± 0.38 | 07.82 ± 0.14 | 08.31 ± 0.31 | 07.28 ± 0.17 | 06.49 ± 0.87 | 05.31 ± 0.13 | 04.23 ± 0.29 |
| 4 | 6.8 | 45.62 ± 0.97 | 49.63 ± 1.56 | 52.79 ± 1.02 | 58.98 ± 0.12 | 51.14 ± 1.13 | 46.28 ± 0.21 | 55.38 ± 0.46 | 49.12 ± 0.74 |
| 6 | 6.8 | 51.31 ± 1.05 | 55.19 ± 0.53 | 67.28 ± 0.34 | 69.41 ± 1.09 | 62.18 ± 0.93 | 59.67 ± 1.41 | 63.76 ± 0.18 | 56.17 ± 1.32 |
| 8 | 6.8 | 65.78 ± 0.41 | 68.47 ± 0.28 | 79.13 ± 0.03 | 81.29 ± 0.97 | 79.64 ± 0.49 | 67.49 ± .0.38 | 79.68 ± 0.97 | 66.87 ± 0.43 |
| 10 | 6.8 | 79.54 ± 0.03 | 81.55 ± 0.78 | 90.28 ± 0.42 | 92.14 ± 0.42 | 89.49 ± 0.31 | 79.45 ± 0.17 | 82.16 ± 0.41 | 79.38 ± 0.19 |
| 12 | 6.8 | 86.71 ± 0.87 | 89.64 ± 0.17 | 94.54 ± 0.69 | 98.36 ± 1.67 | 92.71 ± 0.21 | 87.21 ± 0.29 | 90.56 ± 0.38 | 88.15 ± 0.48 |
Table 5: Cumulative % drug release. Values are expressed as mean ± S D., n=3.
In vitro drug release study and release kinetics
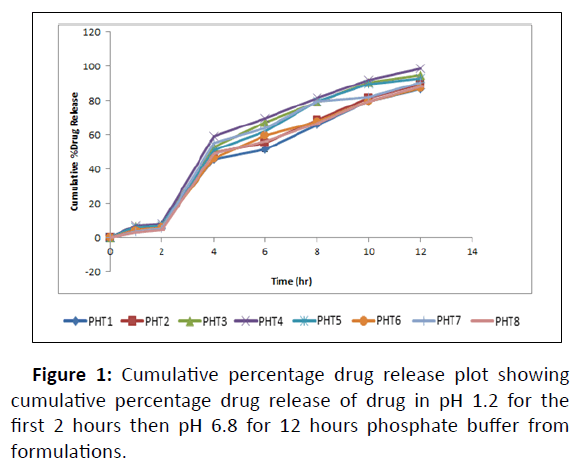
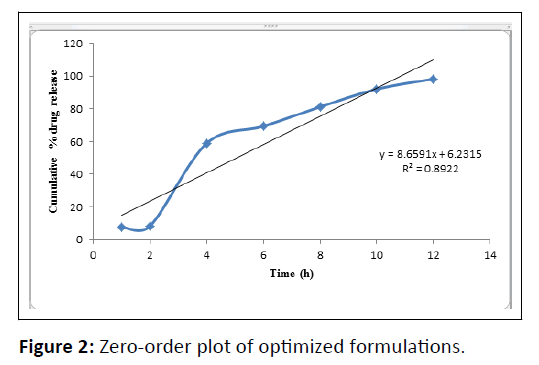
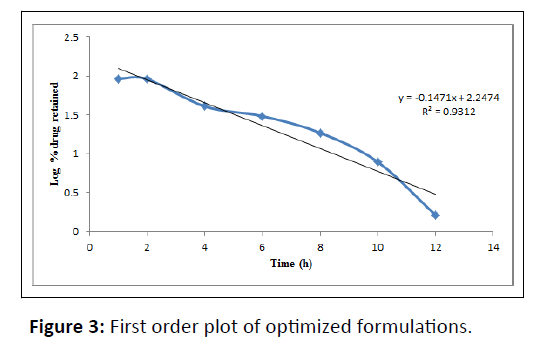
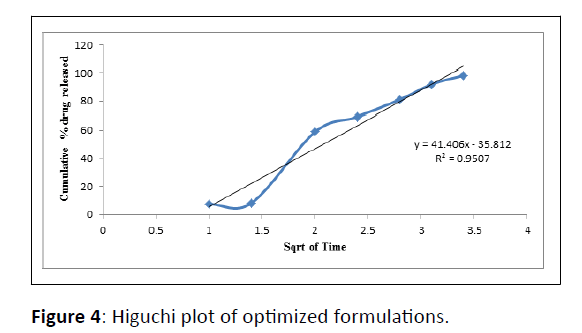
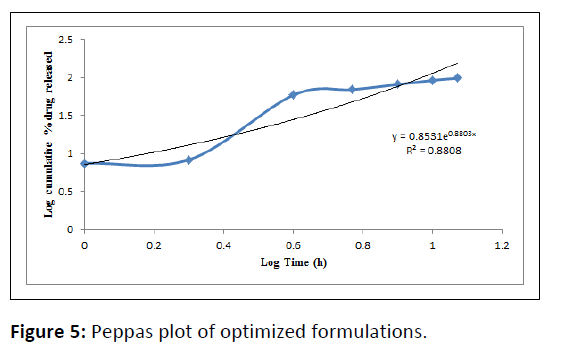
In vitro drug release study of all the developed formulations were tested utilizing a paddle-type tablet dissolving apparatus (USP I type) spinning at 50 rpm. The study was performed in phosphate buffer pH 1.2 for the first 2 hour and then it was replaced by phosphate buffer 6.8 pH and the paddle was rotated continuously for upto 12 hours dissolving medium was kept at 37 ± 0.5â??. Samples were withdrawn at definite intervals, diluted appropriately, and evaluated for cumulative drug release using a UV-Visible spectrophotometer at 275 nm. The proportion of propranolol hydrochloride dissolved in buffer was determined, and the graph was plotted as percent cumulative drug release vs time as shown in Table 6 (Figure 1,2,3,4,5) [17].
| Formulation code | Zero-order | First-order | Higuchi | Korse Meyer-Peppas | |
|---|---|---|---|---|---|
| r2 | r2 | r2 | r2 | N | |
| PHT 4 | 0.8922 | 0.9312 | 0.9507 | 0.8808 | 0.8803 |
Table 6: Drug release kinetics.
Release kinetics studies
In-vitro release data was applied to kinetic models such as zero-order (cumulative percent drug release vs. time), first-order (log mean percent drug unreleased vs. time), Higuchi (mean percent cumulative drug release vs. square root of time) and Korsmeyer-Peppas (log mean percent cumulative drug release vs. log time) to study the release kinetics and mechanism [18].
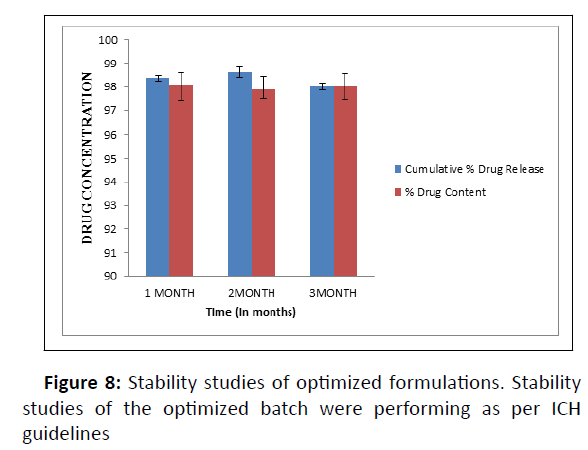
Stability studies
The investigation was carried out on a stability control chamber (Remi Instruments Ltd.) at 40°C/75% Relative Humidity (RH) for 3 months of the optimized formulation. The sample was put in an accelerated stability chamber after being wrapped in coated aluminum foil. Inspections took place at predetermined intervals of 1, 2, and 3 months. The tablets were tested for a variety of physicochemical properties [19, 20].
Results and Discussion
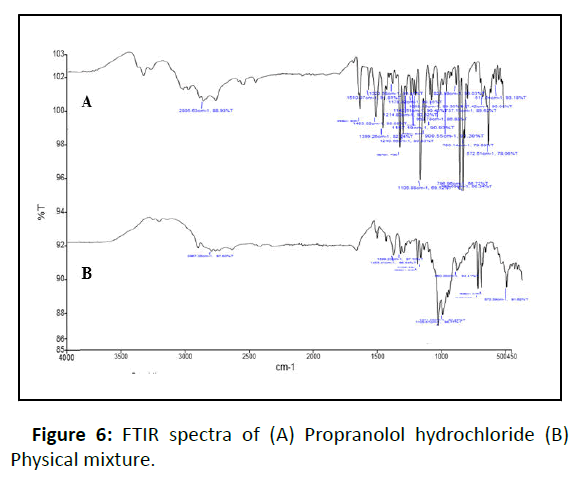
The obtained IR spectra of propranolol hydrochloride show peaks at 2805 cm-1 which correspond to a secondary amine group at 3300 cm-1 due to the hydroxyl group (secondary) at 1105 cm-1 due to the aryl alkyl ether stretching band and a peak at 796 cm-1. However, additional peaks were absorbed in physical mixtures which could be due to the presence of polymers and indicated that there was no chemical interaction between propranolol hydrochloride and other excipients which are shown in (Figure 6).
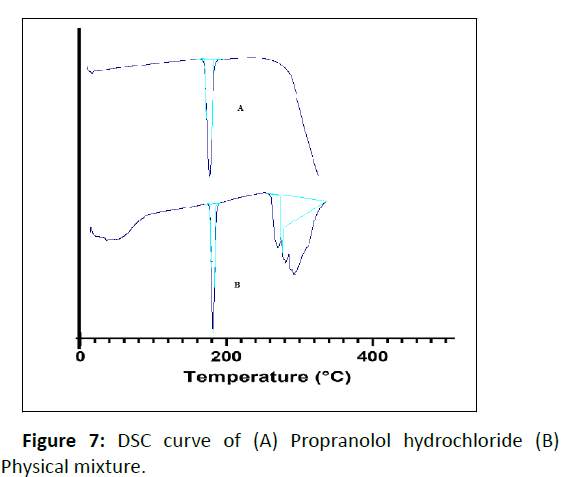
The DSC thermogram of the pure drug (propranolol hydrochloride) displayed an endothermic peak of 169°C corresponding to its melting point and DSC thermograms of the physical mixture of drug and excipients showed the melting peak of the drug at 167.85°C (Figure 7).
Before compression powder blend was evaluated for their characteristic parameters such as angle of repose, bulk density, tapped density, compressibility index, and hausner’s ratio precompression parameters like the angle of repose was found to be within the range of 30.87 ± 0.12 to 34.56 ± 0.73 which indicate the good flow ability. The bulk density of the powder is an important parameter in the compressibility of the powder. The bulk density was found between 0.47 ± 0.01 to 0.50 ± 0.02 gm/cm3. The tapped density was found between 0.551 ± 0.05 to 0.569 ± 0.04 gm/cm3. Carr's index is an indicator of compressibility. A value below 21% shows fair to passable compressibility. It was found to be 11.43 ± 0.64 to 13.78 ± 0.42. Hausner's ratio is another parameter indicating the flow properties. The value of a ratio below 1.25 indicates good flow while above 1.25 indicates poor flow. It was found to be 1.10 ± 0.06 to 1.13 ± 0.18.
The table VI-C mentioned the availability of the system through the year. This is why Google have a downtime less than 5.26 minutes throughout the year because they are running their platforms on kubernetes engine; if there any node fails to respond or destroyed still they platforms is up for use and in the same with the increase of users there platforms are even working similar as it is using by one user. While on other hand many giant IT companies have a downtime greater than 10 days throughout like Microsoft because they are still running their many platforms in a centralized way.
Hydroxypropyl methylcellulose K4M and ethyl cellulose was used in combination to get the enhanced effect for sustained release. PHT1, PHT2 exhibited 86.71%, 89.64% release due to an increase in the concentration of ethyl cellulose. The formulation PHT3, PHT4, PHT5 shows drug release 94.54%, 98.36%, 92.71%. Because of the increase in the concentration of hydroxypropyl methylcellulose K4M and ethyl cellulose in the formulation it will give an increased combined effect.
PHT6, PHT7, PHT8 showed less drug released 87.21%, 90.56%, 88.15% over 12 hours, due to an increase in the concentration of hydroxypropyl methylcellulose K4M in the formulation and decreased concentration of ethyl cellulose. The optimized formulation PHT4 showed a higher rate of drug release up to 12 hours as compared to the other formulation. Due to the combined effect of HPMC-K4M with hydrophilic effect and ethyl cellulose with hydrophobic effect to get an improved rate of drug release.
The optimized formulations PHT4 follows the Higuchi model with non-fickian diffusion based on the regression coefficient of the kinetics data of cumulative drug release from the dosage form. The stability study of the optimized batch was performing as per ICH guidelines and when put in a stability chamber at temperature and humidity conditions of 40°C/75% RH reveals no significant changes in physical parameters, drug content, and dissolution over a time of three months (Figure 8).
The present study has been a satisfactory attempt to formulate a sustained-release drug delivery system of propranolol hydrochloride, an orally administrated antihypertensive drug with improves its oral bioavailability and giving sustained release of the drug for a prolonged period.
Bilayer tablets have been successfully developed and optimized using a direct compression method. Sustained-release propranolol hydrochloride layer was formulated using a combination of hydrophilic and hydrophobic polymers and immediate-release was developed using Kyron T-314. Bilayer tablets Showed good physicochemical attributes and were found to be stable under accelerated stability conditions. The combination of Hydroxypropyl methylcellulose K4M and ethylcellulose successfully controlled the release of propranolol hydrochloride from the bilayer tablet. When concentration of ethylcellulose increases then its shows less drug release similarly on increases hydroxypropyl methylcellulose K4M concentration drug release decreases but when we took both hydroxypropyl methylcellulose K4M and ethylcellulose in equal quantity then it shows adequate drug release. A suggesting higuchi model for drug dissolution profile was approved via combining both hydroxypropyl methylcellulose K4M and ethylcellulose and on increasing the concentration of both the components the release rate also increases. The formulation can be subjected to pharmacokinetic studies and clinical trials in the future for better management of hypertension medication and enhancing patient compliance.
Conclusion
In conclusion, the study was a successful attempt to develop a sustained-release drug delivery system for propranolol hydrochloride, an orally taken hypertension medication, with the goal of enhancing oral bioavailability and ensuring long-term drug release. Several polymers, including HPMC-K4M and ethylcellulose, could be employed to construct a sustainedrelease drug delivery system for propranolol hydrochloride using the direct compression method. The tablets were able to deliver the medication throughout a 12-hour period.
References
- Dandare MS, Sarage RD, Bhaskaran S (2012) Bilayer tablet: A novel approach for the immediate release of telmisartan and hydrochlorothiazide combination. Int J Pharm Tech 4(1): 3970-83.
- Mukhopadhyay S, Goswami L, Satheesh Madhav NV, Upadhyaya K (2010) Formulation and evaluation of floating bioadhesive tablets of ciprofloxacin hydrochloride by direct compression technique. Int J Pharm Pharm Sci 2(3): 113-5.
- Kumar KK, Mahesh M, Sasikanth K (2010) Design development and characterization of sustained release of metformin and gliclazide bi-layered tablets. Int J Bio-Pharm 1(2): 67-71.
- Medicine Net.in. [Last /accessed on 10 Feb 2013].
- Derle D, Joshi O, Pawar A, Patel J, Jagadale A (2009) Formulation and evaluation of buccoadhesive bi-layer tablet of Propranolol hydrochloride. Int J Pharm Pharm Sci 1(1): 206-12.
- Cid E, Mella F, Lucchini L, Carcamo M, Monasterio J (1986) Plasma concentrations and bioavailability of propranolol by oral, rectal, and intravenous administration in man. Biopharm Drug Dispos 7: 559-66.
[Crossref],[Google Scholar],[Indexed]
- Ray D, Prusty AK (2010) Designing and in-vitro studies of gastric floating tablets of tramadol hydrochloride. Int J Appl Pharm 2(4): 12-16.
- Ramakant J, Garud N (2021) Development, optimization and characterization of flurbiprofen matrix transdermal drug delivery system using box–behnken statistical design Future Journal of Pharmaceutical Sciences 7(1): 1-18.
- Wasim A , Garud N (2021) Design expert as a statistical tool for optimization of 5-ASA-loaded biopolymer-based nanoparticles using Box Behnken factorial design. Future Journal of Pharmaceutical Sciences 7(1): 1-17.
- Patric SJ (2006) Martin’s physical pharmacy and pharmaceutical sciences edition 6 wolters kluwer 442-467.
- Goodman & Gilman's. The pharmacology basis of therapeutics. McGraw-Hill medical publishing division London P: 1884.
- Troy DB, Beringer P, Remington (2006) The science and practice of pharmacy. Lippincott Williams & Wilkins.
- Carter SJ (1986). New Delhi: CBS Cooper and gunn’s. Tutorial pharmacy publishers and distributors 220-225.
- Cooper J, Gunn C (1986) Powder flow and compaction. Tutorial pharmacy New Delhi India CBS publishers and distributors. 6: 211-233.
- Patel DM, Prajapati DG, Patel NM (2007) Indian pharmacopeia. ed. Delhi: The controller of publications, Government of India, Ministry of health and family welfare.
- Thorat R, Patil P, Aage R, Puranik P, Salve V (2013) Formulation development and evaluation of venlafaxine HCl sustained release matrix tablet. Int J Pharm Pharm Sci 5(3).
- Indian Pharmacopeia (2007) Ed. Delhi: The controller of publications, Government of India, Ministry of health and family welfare.
- Hadi MA, Rao NGR, Rao SA (2014) Matrix-mini-tablets of lornoxicam for targeting early morning peak symptoms of rheumatoid arthritis. Int J Med Sci17: 357-369.
- Kanvinde SA, Kulkarni MS (2005) Stability of oral solid dosage forms–A global perspective. Pharma times 37(5): 9-16.
- Cartensen JT (1995) Drug stability principles and practices (2nd edtn.). Marcel Dekker Inc.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences