Isolated Middle Cerebral Artery Stenoses: A Serial Follow-up Study using Transcranial Doppler Ultrasonography
Jae Guk Kim, Hanna Choi, Do-Hyung Kim, Sung-Yeon Sohn and Soo Joo Lee
Department of Neurology, Eulji University Hospital, Eulji University, School of Medicine, Daejeon, Republic of Korea
- *Corresponding Author:
- Soo Joo Lee
Department of Neurology, Eulji University Hospital, Eulji University
School of Medicine Dunsanseo-ro 95, Seo-gu, Daejeon, Republic of Korea
Tel: +82-42-611-3430
Fax: +82-42-611-3858
E-mail: sjoolee@eulji.ac.kr
Received date: August 22, 2016; Accepted date: September 28, 2016; Published date: October 04, 2016
Citation: Kim JG, Choi H, Kim DH, et al. Isolated Middle Cerebral Artery Stenoses: A Serial Follow-up Study using Transcranial Doppler Ultrasonography. Stroke Res Ther. 2016, 1:1.
Abstract
Isolated Middle Cerebral Artery Stenoses: A Serial Follow-up Study using Transcranial Doppler Ultrasonography
Background: Whereas the natural history of extracranial arterial disease is well known, the course of intracranial vascular lesions has not been well investigated. We prospectively evaluated the prognosis and long-term course of isolated middle cerebral artery (MCA) stenosis by using transcranial Doppler ultrasonography (TCD).
Methods: Forty-seven MCA stenoses in 32 patients were assessed at entry by TCD, MR angiography and/ or conventional angiography. We excluded patients with potential cardiac embolic source. We also excluded patients who had atherosclerotic lesions in the other intracranial arteries and proximal internal carotid arteries to select isolated MCA stenosis. Fifteen patients had bilateral MCA stenoses and 12 had only asymptomatic MCA lesions. All patients were treated with antiplatelet agents (n=26) or anticoagulants (n=6). Follow-up TCD was done at 3 to 12 month intervals.
Results: The mean duration of follow-up was 29.7 months (3-70 months). During follow-up, new cerebral ischemic events developed in only 2 patients with initially symptomatic stenosis. Progression and regression on follow-up TCD were found more often in symptomatic lesions than in asymptomatic ones (12/20 vs. 5/27, p<0.01). Progression was observed more often in patients with bilateral stenoses than those with unilateral lesions (4/15 vs. 0/17, p<0.05). Focal short segmental stenoses on angiographies showed more regression than diffuse long segmental lesions (11/13 vs. 2/34, p<0.001).
Conclusions: These findings suggest that isolated MCA stenoses are dynamic lesions. Symptomatic stenosis seems to be relatively unstable with a higher frequency of progression and regression than asymptomatic one. Regression of stenosis in some cases may be due to partial lysis of lodged emboli.
Keywords
Middle cerebral artery; Stenosis; Transcranial Doppler ultrasonography (TCD); Progression; Regression
Introduction
Intracranial atherosclerosis may be responsible for approximately 10% of all ischemic strokes in Caucasian patients and is more frequently found among Asian, Hispanics, and African Americans patients [1-7]. The Joint Study of Extracranial Occlusions reported a frequency of middle cerebral artery (MCA) stenosis of 7.6% in large groups of stroke patients in who intra-arterial contrast angiography had been performed [8]. But its actual prevalence has probably been underestimated because invasive vascular imaging has been used to diagnose intracranial stenosis. Intracranial stenotic lesions such as MCA stenosis carry the danger of recurrent cerebral ischemic events, but have not been studied as extensively as either extra- or intracranial internal carotid artery (ICA). Most studies have been retrospective and included only small number of individuals MCA stenosis which was found less frequently than MCA occlusion from either embolic or atherosclerotic occlusion. Currently, secondary prevention is empirical and knowledge about the natural history of this disease derives from a few retrospective studies [9-13]. Therapy to address intracranial stenosis, namely antithrombotic agents and potentially angioplasty, is being investigated [9,14].
Although conventional angiography is still considered standard examination to detect and quantify intracranial stenosis, serial angiographic follow-up studies have some limitations due to invasiveness. Transcranial Doppler ultrasonography (TCD) is a reliable alternative in diagnosing and monitoring MCA stenosis and overcomes the main problems of angiography as its noninvasive nature allows it to be used in patients with MCA stenosis [15-17]. TCD used in combination with MR angiography (MRA) or CT angiography can avoid false positive results [18,19]. We designed a prospective long-term follow-up study to evaluate prognosis and to monitor changes in isolated MCA stenosis on TCD.
Methods
We selected and prospectively followed patients with isolated MCA stenosis to assess clinical occurrence of new ischemic event and the change of lesion on TCD. Thirty-two patients with isolated MCA stenosis detected by TCD and angiography were included among patients who had visited Eulji University Hospital between January 2011 and December 2013. All patients underwent clinical examination, blood and coagulation tests, 12- lead electrocardiography, and brain MRI. All patients had MRA and/ or conventional angiography (CA). Vascular risk factors, i.e. arterial hypertension, diabetes mellitus, hyperlipidemia, cigarette smoking, and cardiac disease were assessed. Patients with history of potential cardioembolism were initially excluded. Echocardiographic studies were done in 20 patients and 24- hour Holter monitoring was also done in 6. Carotid duplex was done in 6 patients. We reviewed angiographic findings on MRA and/ or CA and excluded the patient who had stenosis more than 30% in extracranial ICA and coexisting atherosclerotic stenotic lesion in other intracranial arteries (intracranial portion of ICA, anterior cerebral artery (ACA), and intracranial portion of vertebral artery, basilar artery, and posterior cerebral artery (PCA)) to select patients with isolated MCA stenosis. We also excluded patients with nonatherosclerotic causes of intracranial stenosis (moyamoya disease, vasospasm, meningitis, or arterial dissection).
Stenotic lesion of MCA was classified as being symptomatic or asymptomatic; we considered an MCA stenosis as being symptomatic when the CT or MRI showed an infarct ipsilateral and distally to the vascular territory of the MCA. When the clinical event was a transient ischemic attack (TIA), we considered an MCA stenosis as being symptomatic only if the transient symptoms could clearly be attributed to the affected MCA territory.
The MCA stenosis signal as defined by previously published criteria was detected on the initial TCD recording [15]. We performed TCD studies using a power M-mode Doppler TCD (PMD 150, Spencer Technologies Inc., Seattle, WA) with transtemporal insonation depth of 45 to 60 mm. We diagnosed MCA stenosis when, at a typical location of the artery, segmental flow acceleration together with disturbance in the frequency spectrum of the arterial signal was found on TCD exam. The criteria for diagnosis were as follow: circumscribed increased mean flow velocity (MFV) >80 cm/sec, side-to-side difference of > 30 cm/sec, poststenotic reduced flow signal, and the disturbance of frequency spectrum [15]. Bilateral MCA stenoses were assumed if the sign of disturbance of frequency spectrum was combined in patients with MFV >80 cm/sec in both MCAs. MRA or CA was additionally performed, confirming the TCD diagnosis at the beginning of the observation period. A three-point grading system was employed for classification of stenosis on MRA or CA: mild (1-49%), moderate (50-69%), and severe stenosis (70-99%) [2,18]. If a flow void was observed on MRA images the stenosis was graded as severe stenosis [18]. The smallest diameter of each artery was visually compared to the diameter of the nearly normal arteries [20]. We reviewed the extent of the stenotic lesion on angiography. We classified the morphology of stenotic lesions into two lengths of the stenotic segment; short segment-involved lesion and long segment-involved one. We defined long segment-involved lesion if the stenosis included more than 1/3 of the entire length of MCA stem (M1 segment) arbitrarily.
Twenty-six patients were treated with antiplatelet agents and 6 with anticoagulants. Each neurologist in charge decided treatment with either antiplatelet agent or anticoagulant. Follow-up interview of patients and their families, neurological examination and TCD were done at 3 to 12 month intervals. We followed the patients using the same ultrasound equipment during follow-up. The change of MCA stenosis during the follow-up period was categorized as ‘progression’, ‘unchanged’, or ‘regression’ if at least 25% change of MFV was detected when it was compared with the results of previous studies. MCA occlusion is indicated by the absence of a TCD signal from the insonation depths at which an MCA signal is typically obtained, in association with the presence of ACA and PCA signals from the temporal window [15].
To compare the categorical variable between two groups, two-tailed Fisher exact test was used with a significance value at the p<0.05 levels.
Results
Demographic characteristics of the study population and vascular risk factors are shown in Table 1. Twenty patients had symptomatic MCA stenoses that were considered responsible for symptoms. The other 12 had asymptomatic lesions. Among 20 patients with symptomatic MCA stenosis 15 patients presented with TIA and the remaining 5 had minor strokes. Eleven patients had previous or preceding TIAs. Ten out of 11 patients presented with recurrent TIAs at least two times with stereotypic same pattern. In 11 patients with presumed asymptomatic MCA stenosis the reasons of evaluation were as follow: nonspecific headache and dizziness in 4 patients, vertigo in 3, and syncope in 1. The other 3 patients had a stenotic lesion contralateral to symptoms related side.
| Clinical Characteristics | Values |
|---|---|
| Age, years (range) | 53.7±9.88 (34-68) |
| Sex (F), n (%) | 7 (21.8) |
| Smoker, n (%) | 12 (37.5) |
| Medical History, n (%) | |
| Hypertension | 16 (50) |
| Diabetes | 6 (18.8) |
| Hyperlipidemia | 10 (31.3) |
| Medication, n (%) | |
| Anticoagulation | 6 (18.8) |
| Antiplatelet agent | 26 (81.2) |
| Symptom related to stenosis, n (%) | |
| TIA | 15 (46.9) |
| Minor stroke | 5 (15.6) |
| Time lapse (onset to TCD test), days | 46.7±75.8 (1-333) |
| Time lapse (onset to angiography), days | 8.9±11.0 (0-42) |
Table 1: Characteristics of the Study Population.
MRI showed ischemic lesions in a vascular territory corresponding MCA stenosis in 13 patients (striatocapsular infarct in 4 patients, small basal ganglia or capsular lesion in 6, and lesion of borderzone white matter area in 3). There was no patient who had infarction of cortical involvement on MRI.
Forty-seven MCA stenotic lesions were detected by TCD and angiography at entry. The mean time lapse between initial TCD and angiograhy was 8.9 days (range, 0 to 42 days). Table 2 shows baseline characteristic data of MCA stenoses on TCD and angiography. The number of symptomatic MCA stenosis arteries was 20, and that of asymptomatic stenoses 27. Unilateral MCA stenosis was found in 17 patients and bilateral MCA stenoses in 15. Stenosis was confirmed by MRA in 25 patients, by CA in 1, and by both CA and MRA in 6. Severe stenosis on angiography was observed in 9 (19.1%) arteries, moderate in 14 (29.8%), and mild in 24 (51.1%). Thirteen stenotic lesions were characterized as short segment involvement and the other 34 had long-irregular stenosis on MRA and/or CA. The mean initial MFV of MCA and the degree of stenosis on angiography was higher in symptomatic stenotic lesions than in asymptomatic ones (Table 2).
| Symptomatic (n=20) | Asymptomatic (n=27) | Unilateral (n=17) | Bilateral (n=30) | |
|---|---|---|---|---|
| Mean MFV on TCD, cm/sec (range) |
163±64 (82-282) | 110±26 (81-187) | 132±54.4(81-256) | 132±53.5(81-282) |
| Degree of stenosis on angiography severe (n=9) moderate (n=14) mild (n=24) |
8 8 4 |
1 6 20 |
4 4 9 |
5 10 15 |
| Shape of stenosis on angiography short long |
11 9 |
2 25 |
7 10 |
24 30 |
Table 2: Characteristics of MCA stenotic lesions: TCD and Angiographic Findings.
The mean duration of follow-up was 29.7 months (3-70 months). The number of total TCD exams was 108 and the mean number of TCD exams per patient was 3.4 (range 2 to7). During follow-up, new cerebral ischemic events (TIA n=1, stroke n=1) developed in only two patients, each with initially symptomatic MCA stenosis. One patient had treated with anticoagulation and the other with antiplatelet agent. The first patient was a 40 year-old man with a history of recurrent TIAs. Initial TCD showed markedly increased MFV of 256 cm/sec in left MCA and conventional angiography also showed severe (>70%) short segment-involved stenosis. TIA developed again 3 months later despite anticoagulation treatment. On follow-up TCD MFV of MCA decreased to about 70% of the initial MFV. But follow-up MRA did not show significant change of the preexisting MCA stenosis. The other patient was a 69 years-old woman. Her initial event was also a TIA. Initial TCD exam showed increased MFV of left MCA stem and stenosis of moderate degree (signal loss more than 50%) showing long segment-involved irregular shape on MRA. One year later sudden right hemiparesis developed and MRI showed newly developed small infarction involving the striatocapsular area. Follow-up TCD and MRA did not show any significant change compared with previous study.
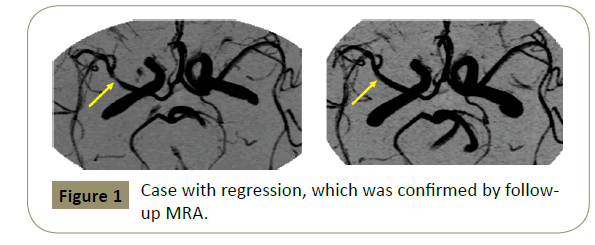
The relation of the characteristics of MCA stenoses with follow-up TCD results is shown in Table 3. Overall follow-up TCD exams showed progression in 4 middle cerebral arteries and regression in 13. Progression was found more often in symptomatic lesions than in asymptomatic ones (3/20 vs. 1/27, p=0.17) but it did not reach a statistical significant difference. Regression was also found more frequently in symptomatic lesions than in asymptomatic ones (9/20 vs. 4/27, p<0.05). Significant changes, either progression or regression on follow-up TCD, was found more often in symptomatic lesions than in asymptomatic ones (12/20 vs. 5/27, p<0.01). Progression was observed more often in patients with bilateral stenosis than in those with unilateral lesions (3/15 vs. 0/17, p<0.05). And focal short segment-involved stenotic lesions on angiography showed more regression than diffuse long segmental lesions (11/13 vs. 2/34, p<0.001). Progression on TCD developed in 4.2% (1/24) of mild stenosis on angiography, in 7.1% (1/14) of moderate ones, and in 22.2% (2/9) of severe ones. Regression developed in 20.8% (5/24) of mild stenosis on angiography, in 28.6% (4/14) of moderate ones, and 44.4% (4/9) of severe ones. Progression and regression more frequently developed in MCA stenosis of severe degree. Progression or regression developed in 75% (6/8) of symptomatic stenosis with severe degree, in 50% (4/8) of symptomatic ones with moderate degree, and in 50% (2/4) of symptomatic ones with mild degree. No change developed in an asymptomatic stenosis of severe degree. Progression or regression developed in 16.7% (1/6) of asymptomatic arteries with moderate degree and in 20% (4/20) of asymptomatic arteries of mild degree. We had no patient who progressed to complete occlusion on followup TCD. We did not always perform CA or MRA again in patients who showed any changes (regression or progression) during the follow-up period. We confirmed follow-up angiographic findings in 6 of 17 arteries, which showed regression (Figure 1) or progression on follow-up TCD exams.
| Nature of lesions | Symptomatic n=20, (%) |
Asymptomatic n=27, (%) |
Unilateral n=17, (%) |
Bilateral n=30, (%) |
Short n=13, (%) |
Long n=34, (%) |
|---|---|---|---|---|---|---|
| Progression | 3 (15) | 1 (3.7) | 0 (0) | 4 (13.3) | 1 (7.7) | 3 (8.8) |
| Regression | 9 (45) | 4 (14.8) | 7 (41.2) | 6 (20) | 11 (84.6) | 2 (5.9) |
| No change | 8 (40) | 22 (81.5) | 10 (58.8) | 20 (66.7) | 1 (7.7) | 29 (85.3) |
Table 3: Relation the characteristics of stenosis with TCD follow-up results.
Discussion
Autopsy series of Caucasians show a low prevalence of isolated stenosis or occlusion of the MCA. Most are the results of embolism from the heart or its valves, or tandem lesions occurring in association with carotid bifurcation atherosclerosis. Lhermitte et al. [21] in postmortem exams of patients with MCA territory infarction found that solitary MCA atherosclerosis was present in only 4% of the total patients. Intracranial MCA occlusive disease has been found more frequently in Asians like our patient population compared to Caucasians [1-7]. Although our data derived from a single hospital, the prevalence of isolated MCA stenosis in this study seems not to be high. We were able to include only 32 patients with isolated MCA stenosis among about 4000 patients who visited our TCD laboratory for 3 years.
The published data about the clinical occurrence of MCA stenosis have been small, potentially limiting ability to draw definite conclusions [22-29]. An early study of prognosis in patients with MCA stenosis was that of Hinton et al. [22] In 16 patients with angiographically demonstrated MCA stenosis the prognosis was remarkably good under anticoagulation. Feldmeyer et al. [23] reported two TIAs and one stroke among 13 patients during 59 months. However, Croton et al. [24] reported an opposite experience among their 21 patients with angiographically demonstrated MCA stenosis. They found recurrent TIA in 2 patients, and fatal stroke in 4 out of 21 during a 7-year follow-up period. The largest data with regard to the prognosis of patients with MCA stenosis and occlusion came from the extracranial-intracranial (EC/IC) bypass study [13] Bogousslavsky et al. [13] followed 85 patients over 4 months. They observed an annual rate of 5.8% for TIA and the same rate for recurrent stroke distal to the affected MCA, resulting in a considerably higher recurrence rate of ischemia than the other series. In this study two patients of 32 had new ischemic events during a mean follow-up 29.7 months. The rate of annual recurrence was approximately 2.5%. Compared with previous studies above the rate of recurrence was not high.
There have been several reports about the lesion progression for MCA stenosis (Table 4) [25-29] Becker et al. [25] followed up 8 patients with symptomatic isolated MCA stenosis for mean 50 months. They found regression in 4 patients and progression in one. Kuehen et al. [26] found that progression and regression developed more often in symptomatic stenoses (21%) than in asymptomatic ones (7%) during mean follow-up periods of 28.5 months. Arenillas et al. [27] reported their results that 32.5% MCA stenosis progressed, 7.5% regressed, and 60 remained stable on TCD with median follow-up of 22.55 months. In study derived from Chinese population, 14 (13.1%) patients of 107 had clinical events during 6-month period and follow-up TCD showed regression in 35 (32.7%) patients and progression in 15 (14.1%) [28]. In Korean study, 12.6% MCA stenosis progressed, 87.4% stationary or regressed on TCD with follow-up with 6 months [29]. The results above showed the discrepancy among previous studies. The frequency of lesion progression in our results is similar with that of study in Chinese patients [28].
| Author | N | Diagnosis at entry |
NA (symptomatic/ asymptomatic) |
Follow-up periods, months | Clinical Occurrence | Progression/Regression on follow-up TCD |
|---|---|---|---|---|---|---|
| Berker et al.[25] | 8 | TCD/ angiography | 8 (8/0) |
50 | 0 | 1(12.5%) / 3(37.5%) |
| Kuehen et al.[26] | 102 | TCD/ angiography | 122 (52/70) |
28.5 | 6 | Unchanged - 93%/ Progression and regression -21% of symptomatic stenoses -7% of asymptomatic stenoses |
| Arenillas et al.[27] | 40 | TCD/ angiography | 40 (40/0) |
26.5 | 8 | 13(32.5%) / 3(7.5%) |
| Wong et al.[28] | 107 | TCD | 107 (107/0) |
6 | 14 | 13(12.1%) / 35 (32.7%) |
| Jeon et al.[29] | 103 | TCD/MRA | 103 (66/37) |
6 | - | Progression - 13 (12.6%) Stable or improved - 90 (87.4%) |
| Present study | 32 | TCD/ angiography | 47 (20/27) |
29.7 | 2 | 4(8.5%) / 13(27.7%) symptomatic stenosis - 3(15%) / 9(45%) |
Table 4: Previous Studies about Monitoring of MCA stenosis using TCD in the literatures.
It is generally accepted that MCA stenosis is commonly due to atherosclerosis. However, certain findings of our study support the hypothesis that, at least during the acute phase of stroke, a significant number of MCA stenoses may have an embolic rather than atherosclerotic origin. Regression of stenosis in some cases may be due to partial lysis of lodged emboli [30-32]. Embolic occlusion of the MCA is frequent and could mimic atherosclerotic stenosis in ultrasound studies as well as imaging studies, when recanalization has started. When we reviewed the time interval between symptom onset and the first TCD exam in 20 patients with symptomatic stenosis, the number of patients in whom initial TCD was done within 10 days after symptom onset was 7. Out of these 7 patients, 5 showed regression on follow-up TCD. No definite criteria exist for in vivo discrimination of stenosis due to atherosclerosis versus incomplete recanalization after embolic occlusion if initial serial angiography or ultrasound examinations are not performed to detect revacularization after an initial occlusion. No microembolic signal was identified in a recent study during long-term chronic MCA stenosis TCD monitoring, suggesting the clinical recurrence is mainly caused through hemodynamic mechanisms [33,34]. Some angiographic finding could be helpful to differentiate between two types of stenosis. Angiography revealed a short or focal MCA stenosis thought to be more suggestive of incomplete recanalization than of atherosclerotic stenosis. But stenoses with diffuse long and bilateral involvement are most likely explained by intrinsic atherosclerotic disease. Our results showed that regression developed more often in stenotic lesions with focal short segment involvement than in MCA stenosis with diffuse long segment narrowing.
If two or even more stereotyped ischemic events occur, the clinical course is considered atypical in patients who had documented MCA embolization. When we reviewed clinical symptoms with other data, 7 patients of 10 with history of preceding more than two stereotypic TIA had stenosis of severe degree on angiography. This finding suggests possibility of hemodynamic low flow status due to severe stenosis. Although the distribution of stenosis severity was different between symptomatic lesions and asymptomatic ones, symptomatic lesions showed a higher frequency of progression and regression than asymptomatic ones in each grade of stenosis severity.
MCA stenosis could cause ischemia of supplied distal territories, including cerebral cortex, by impairment of distal perfusion and by the release of emboli mentioned above. Alternatively the other pathogenetic mechanism is that silent atherosclerosis of MCA stem might become apparent by blockage of the orifice of the deep penetrating lenticulostrate arteries, causing small deep infarct [35,36]. This explanation may be supported by the fact that MRI showed no evidence for other site of small-vessel disease and cortical infarction in our patients.
This study has potential limitations. First, the small size of the series and selection from one hospital can diminish the significance of our findings. Second, there have been no validated criteria of MCA progression or regression to TCD. We used criteria of mean velocity changes more than 25% compared with initial exam. Difference of less than 20% in mean flow velocity of these arteries between exams was accepted [37-39]. However, an experimental study has shown that when stenosis reaches a critical severity, blood flow may also be compromised. The reduction in flow volume results in dampened velocity rather than increased velocity [40,41]. We may have possibility to misclassify progression or regression because we did not obtain follow-up vascular imaging in all patients showing regression on TCD. Third is that our initial data showed variable time lapses from the onset to the first TCD in 20 symptomatic cases. This means that data was missing in the acute phase. It also requires the caution to interpret the results. Other limitation is that we did not perform echocardiography in all patients. Especially transesophageal echocardiography, which is more sensitive than the transthoracic echocardiography in the detection of aortic arch atheromatous disease and atrial septal defects, was performed in only 4 patients and this might have resulted in an underestimation of potential embolic sources. Other is difficulty to define the morphology of stenotic artery; short segment and long segment. If a flow signal void was seen on MRA, it was difficult to determine the exact length of the involved segment because MRA has a tendency to overestimate the degree of stenosis. Different medications can be the other limitation. From our results it is not possible to determine the effect of treatment.
In conclusion, isolated MCA stenoses are dynamic lesions. Symptomatic stenosis seems to be relatively unstable with a higher frequency of progression and regression than asymptomatic one. Regression of stenosis in some cases may be due to partial lysis of lodged emboli.
References
- Sacco R, Kargman DE, Gu Q, Zamanillo MC (1995) Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction: the Northern Manhattan Stroke Study. Stroke26:14-20.
- Wityk RJ, Lehman D, Klag M, Coresh J, Ahn H,et al. (1996) Race and sex differences in the distribution of cerebral atherosclerosis. Stroke27:1974-1980.
- Caplan LR, Gorelick PB, Hier DB (1986) Race, sex, and occlusive cerebrovascular disease: a review. Stroke17:648-655.
- Gorelick PB, Caplan LR, Hier DB, Parker SL, Patel D (1984) Racial differences in the distribution of anterior circulation occlusive disease. Neurology34:54-59.
- Feldman E, Dancault N, Kwan E, Ho KJ, Pessin MS, et al. (1990) Chinese-white differences in the distribution of occlusive cerebrovascular disease. Neurology40:1541-1545.
- Wong KS, Huang YN, Gao S, Lam WWM, Chan YL, et al. (1998) Intracranial stenosis in Chinese patients with acute stroke. Neurology50:812-813.
- Huang YN, Gao S, Li SW, Huang Y, Li JF, et al. (1997) Vascular lesions in Chinese patients with transient ischemic attack. Neurology48:524-525.
- Hass WK, Fields WS, Norht RR, Kricheff II, Chase NE, et al. (1968) Joint study of extracranial artery occlusion. II Arteriography, techniques, sites and complication. JAMA203:159-968.
- Chimowitz MI1, Kokkinos J, Strong J, Brown MB, Levine SR, et al. (1995) The Warfarin-Aspirin Symptomatic Intracranial Disease Study Group. The Warfarin-Aspirin Symptomatic Intracranial Disease Study. Neurology45:1488-1493.
- EC/IC Bypass Study Group (1985) Failure of extracranial-intracranial arterial bypass to reduce the risk of ischemic stroke: results of an international randomized trial. N Eng J Med 313:1191-1200.
- Bauer RB, Sheehan S, Wechsler N, Meyer J (1962) Arteriographic study of sites, incidence, and treatment of arteriosclerotic cerebrovascular lesions. Neurology12:698-711.
- Wechsler LR, Kistler JP. Davis KR, Kaminski MJ (1986) The prognosis of carotid siphon stenosis. Stroke17:714-718.
- Bogousslavasky J, Barnett HJM, Fox AJ, Hachinski VC, Taylor W (1986) Atherosclerotic disease of middle cerebral artery. Stroke17: 1112-1120.
- Connors JJ, Wojak JC (1999) Percutaneous transluminal angioplasty for intracranial atherosclerotic lesions: evolution of technique and short-term results. J Neurosurg91:415-423.
- Ley-Pozo J, Ringelstein EB (1990) Noninvasive detection of occlusive disease of carotid siphon and middle cerebral artery. Ann Neurol28:640-647.
- Babikian V, Sloan MA, Tegeler CH, DeWitt LD, Fayad PB, et al. (1993) Transcranial Doppler validation pilot study. J Neuroimaging3:242-249.
- Alexander AV, Demchuk AM, Wein TH, Grotta JC (1999) Yield of transcranial Doppler in acute cerebral ischemia. Stroke30:1604-1609.
- Rother J, Schwartz A, Wentz KU, Rautenberg W, Hennnerici M (1994) Middle cerebral artery stenoses: assessment by magnetic resonance angiography and transcranial Doppler ultrasound. Cerebravasc Dis 4:273-279.
- Wong KS, Lam WM, Liang L, Huang YN, Chan YL, et al. (1996) Variability of magnetic resonance angiography and computer tomography in grading middle cerebral artery stenosis. Stroke27:1084-1087.
- Samuel OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI (2000) A standard method for measuring intracranial arterial stenosis. Am J Neuroradiol21:643-649.
- Lhermitte F, Gautier JC, Derouesne C (1970) Nature of occlusions of the middle cerebral artery. Neurology20:82-88.
- Hinton RC, Mohr JP, Ackerman RH, Adair LB, Fisher CM (1979) Symptomatic middle cerebral artery stenosis. Ann Neurol 5: 152-157.
- Feldmeyer J, Merendez C, Regli F. Symptomatic stenoses of the middle cerebral artery. Rev Neurol (Paris) 1983;139:725-736.
- Croston RN, Kendall BE, Marshall J (1984) Prognosis in middle cerebral artery stenosis. Stroke15:237-241.
- Becker V, Eckert B, Thie A (1996) Isolated symptomatic stenosis of the middle cerebral artery in younger adults. Eur Neurol36:65-70.
- Kuehnen J, Steinke W, Kern R, Artemis N, Hennerici M (1996) Long term course of symptomatic and asymptomatic middle cerebral artery stenosis. Neurology46:2 suppl:A281
- Arenillas JF, Molina CA, Montaner J, Arbilleria S, Gonzalez-Sanchez MA, et al. (2001) Progression and clinical recurrence of symptomatic middle cerebral artery stenosis. A long-term follow-up transcranial Doppler ultrasound study. Stroke32:2898-2904.
- Wong KS, Li H, Lam WWM, Chan YL, Kay R (2002) Progression of middle cerebral artery occlusive disease and its relationship with further vascular events after stroke. Stroke33:532-536.
- Jeon HW, Cha JK (2008) Factors related to progression of middle cerebral artery stenosis determined using transcranial Doppler ultrasonography. J Thromb Thrombolysis25:265-269.
- Adams HP Jr, Gross CE (1981) Embolism distal to stenosis of the middle cerebral artery. Stroke12:228-229.
- Behren S, Cornelius A, Schwartz A, Hennerici M (1998) Spontaneous decrease of MCA stenosis due to cerebral thromboembolism. Cerebrovasc Dis8:362.
- Sliwka U, Klotzsch C, Popescu O, Brandt K, Schmidt P, et al. (1997) Do chronic middle cerebral artery stenoses represent an embolic focus? Stroke28:1324-1327.
- Segura T, Serena J, Castellanos M, Teruel J, Vilar C, et al. (2001) Embolism in acute middle cerebral artery stenosis. Neurology56:497-501.
- Wong KS, Gao S, Lam WWM, Chan YL, Kay R (2001) A pilot study of microembolic signals in patients with middle cerebral artery stenosis. J Neuroimaging11:137-140.
- Lyrer PA, Engelter S, Radu EW, Steck AJ (1997) Cerebral infarcts related to isolated middle cerebral artery stenosis. Stroke28:1022-1027.
- Caplan LR (1989) Intracranial branch atheromatous disease: neglected understudied and underused concept. Neurology39:1246-1250.
- Maeda H, Etani H, Handa N, Tagaya M, Oku N, et al. (1990) A validation study on the reproducibility of transcranial Doppler velocimetry. Utrasound in Med. & Biol16:9-14.
- Totaro R, Marini C, Cannarsa C, Prencipe M (1992) Reproducibility of transcranial Doppler sonography: a validation study. Utrasound in Med Biol 173-177.
- Sorteberg W, Langmoen IA, Lindegaard KF, Nornes H (1990) Side to side differences and day-to-day variations of transcortical Doppler parameters in normal subjects. J Ultrasound Med9:403-409.
- Spencer MP, Reid JM (1979) Quantification of carotid stenosis with continuous-wave (C-W) Doppler ultrasound. Stroke 10:326-330.
- Tegler CH, Babikian VL, Gomez CR (1996) Neurosonlogy. St. Louis: Mosby-Year Book 41.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences