Therapeutic Hypothermia Protects In Vitro Brain Barrier from Ischaemic Damage through Attenuation of Inflammatory Cytokine Release and Apoptosis
Manik Mathur and Ulvi Bayraktutan
Published Date: 2017-05-27Division of Stroke, Clinical Neuroscience, Clinical Sciences Building, The University of Nottingham, United Kingdom
- *Corresponding Author:
- Dr Ulvi Bayraktutan
Stroke, Division of Clinical Neuroscience
Clinical Sciences Building, Nottingham City Hospital Campus
The University of Nottingham, Hucknall Road, Nottingham, UK
Tel: +44(115)8231764
Fax: +44(115)8231767
` E-mail: ombamalu@uwc.ac.za
Received Date: March 25, 2017; Accepted Date: May 24, 2017; Published Date: May 29, 2017
Citation: Mathur M, Bayraktutan U. Therapeutic Hypothermia Protects In Vitro Brain Barrier from Ischaemic Damage through Attenuation of Inflammatory Cytokine Release and Apoptosis. Stroke Res Ther. 2017, 2:1.
Abstract
Background: Ischaemic stroke disintegrates communications within a controlled ensemble of cells that constitutes the blood-brain barrier. Therapeutic hypothermia may act as a neuroprotectant during intra- and post-ischaemic injuries. Hence, this study focuses on the effects of oxygen-glucose deprivation mediated ischaemic injury on the barrier in the absence or presence of hypothermia (35oC).
Methods: An in vitro model of human blood-brain barrier, composed of human brain microvascular endothelial cells, astrocytes and pericytes, was subjected to oxygen-glucose deprivation (OGD) in the absence or presence of hypothermia in a time-dependent fashion. Following treatments, the integrity and function of blood-brain barrier was assessed by transendothelial electrical resistance (TEER) and paracellular flux of high and low molecular weight permeability markers i.e. Evan’s blue albumin (EBA) and sodium fluorescein (NaF), respectively. Levels of pro-inflammatory cytokines were assessed in all three cell lines using specific ELISA-based kits. To study the rate of apoptosis, caspase-3/7 enzyme activities and percentage of DNA fragmented cells (TUNEL) were measured.
Findings: Cells exposed to OGD alone displayed higher levels of both tumor necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β). Concurrent use of hypothermia significantly decreased both cytokine levels in all three cell lines. Furthermore, hypothermia improved the blood-brain barrier integrity and function as ascertained by an increase in TEER and concomitant decreases in paracellular flux of EBA and NaF. Hypothermia also increased the expression of claudin-5 protein during intra-ischaemic phase which may contribute to improved barrier integrity and function. Lastly, intra- and post-ischaemic treatments of endothelial cells and astrocytes with hypothermia effectively reduced the rate of apoptosis as evidenced by decrease in TUNEL-positive cells and capase-3/7 enzyme activities.
Conclusion: Application of hypothermia during intra- and post-ischaemic periods of an ischaemic injury appears to protect cerebral barrier integrity and function.
https://maviyolculuk.online/
https://mavitur.online/
https://marmaristeknekirala.com.tr
https://tekneturumarmaris.com.tr
https://bodrumteknekirala.com.tr
https://gocekteknekirala.com.tr
https://fethiyeteknekirala.com.tr
Keywords
Stroke; Ischaemic injury; Therapeutic hypothermia; TNF-alpha; IL- 1beta; Apoptosis; Claudin-5
Introduction
Blood-brain barrier (BBB) accounts for a selective transition of ions and molecules from cerebral circulation to the brain parenchyma [1]. The BBB is formed by endothelial cells, surrounded by basement membrane and astrocytes, pericytes and neurons, where astrocytes and tight junction proteins are known to significantly contribute to the overall tightness of the BBB [2,3]. However, various cerebral conditions, notably acute ischaemic attack compromises the BBB integrity and thus contribute to ensuing oedema formation [4]. Amongst the cells that make up the BBB, endothelial cells and astrocytes are known to be the potent producers of pro-inflammatory cytokines, notably interleukin- 1β (IL-1β) and tumor necrosis factor-alpha (TNF-α) [5]. As the cytokines may cross the BBB, they have long been implicated in early breakdown of the endothelial barrier [1,6]. Recent studies have demonstrated that diminished expressions of tight junction proteins and reduced BBB-associated cell viability also play major roles in inducing cytokine-induced BBB damage [7,8]. TNF-α is a pleiotropic inflammatory cytokine, usually demonstrates a bi-phasic release pattern [9]. The BBB-associated endothelial cells contain TNF receptor-1 whereas IL-1β which tend to work synergistically with TNF-α at the site of ischaemic injury has a late releasing property usually after 6 to 24 h post-ischaemic attack [9]. IL-1β also induces vasogenic oedema, triggers the endothelium for leukocyte adherence and contributes to influx of calcium [10]. The release of cytokines at the site of injury is mainly followed by chemokines such as monocyte chemoattractant protein-1 and cytokine-induced neutrophil chemoattractant [11]. Apoptosis, an active metabolic and genetically encoded death pathway, constitutes another major mechanism in ischaemiainduced neurovascular injury. Ischaemic signalling pathway may be employed by cytotoxic T cells, whose product, granzyme B, directly cleaves procaspase molecules, thereby activating the caspase enzymes in cells targeted for destruction [12]. The barrier breakdown at high concentrations of cytokines correlates with caspase-3/7 activation via Jun amino-terminal kinases (JNK) and protein kinase C (PKC) signaling pathways [13].
Anatomically, the endothelial cells of the BBB are distinguished from those in the periphery by augmented mitochondrial content, absence of fenestrations and merely pinocytotic activity [14]. The BBB properties are primarily determined by endothelial junctional complexes consisting tight junctions and adherens junctions. Endothelial cells being the main component of the barrier and retains tight junction proteins, i.e. claudin-5 and occludin. These tight junctions between the BBB endothelial cells lead to high endothelial electrical resistance and low paracellular permeability with self-assembled into ‘zip-locked’ structures, forming the physical barrier [15,16]. Following an ischaemic attack, tight junction protein expressions are decreased directly contributing to increased barrier permeability [17].
Restoring perfusion by selectively aiming the occluded artery through intra-arterial thrombolysis or mechanical thromboembolectomy has been beneficial in patients with ischaemic stroke [18]. Therapeutic hypothermia may be an option for neuroprotection due to its putative multi-faceted protective roles given the close associations observed in clinical studies amongst brain temperature, initial stroke severity and infarct volume [19].
Considering the above and the negative impact of temperatures below 35°C on platelet count, coagulation cascade and plasminogen activators [20], the current study has investigated the effects of ischaemic injury mimicked by oxygen-glucose deprivation (OGD) in laboratory settings on an in vitro model of human BBB in the absence or presence of therapeutic hypothermia (35°C).
Material and Methods
Cell culture
Human brain microvascular endothelial cells (HBMEC), human astrocytes (HA) and human pericytes (HP) were purchased from TCS Cellworks (Buckingham, UK) and cultured up to passage 7. While HBMEC and HA were grown in their respective specialised media (Sciencell, Caltag Systems, Buckingham, UK) and HP were cultured using Dulbecco’s Modified Eagle’s Medium (DMEM) in a humidified atmosphere (95% relative humidity) under normal conditions at 37°C with 5% CO2.
Induction of oxygen-glucose deprivation
To study the effects of intra-ischaemic and post-ischaemic injury, the confluent cells were placed in a cell culture incubator (SANYO, MCO-18M Multi-gas cell) before exposure to 94% N2, 1% O2 and 5% CO2 at 37°C. In these experiments, the growth media were replaced with glucose-free RPMI-1640 media (Life Technologies, Paisley, UK). While cells subjected to 4 or 20 h of OGD in the absence or presence of hypothermia mimicked intra-ischaemic treatment regimens, those exposed to 4 h of OGD followed by 20 h of hypothermia mimicked post-ischaemic treatment. Cells cultured under normal conditions served as controls.
In vitro model of human blood-brain barrier
A triple culture model of human BBB composed of HBMEC, HA and HP were set up using transwell inserts (polyester membrane, 12 mm diameter, 0.4 μm pore size, Corning Costar, High Wycombe, UK) [21]. HA were seeded on the basolateral side of polyester membrane. Following overnight adherence, inserts were transferred to 12-well plates containing fresh media and cultured to about 90% confluence. Thereafter, the HBMEC were seeded onto the inner part of the same inserts. Once full confluence achieved in both cell layers, the inserts were then transferred to a fresh 12-well plate containing confluent HP to establish the triple culture model.
Measuring integrity and function of the BBB
The assessment of the integrity and function was carried out by measuring transendothelial electrical resistance (TEER) using an Ohm’s meter and the flux of permeability markers i.e. sodium fluorescein (NaF; 376 Da), and Evan’s blue-labelled albumin (EBA; 67 kDa) across the barrier, respectively. To evaluate leakage across the barrier, either NaF (10 μg/mL) or EBA (165 μg/mL; 0.5 mL) were gently added to the inserts and incubated for 1 hour at 37°C. Following incubation, samples were collected from the lateral and basolateral sides (400 μL). The concentrations of EBA and NaF molecules in the samples were measured by using a plate reader (BMG LABTECH Omega, Aylesbury, UK) at absorbance: 610 nm and a fluorescent plate reader (TECAN GENios, Reading, UK) at excitation: 440 nm, emission: 525 nm, respectively. The flux of permeability markers was calculated as cleared volume by the formula: Cleared volume (μL)=concentration (abluminal reading) × volume (abluminal) × concentration (luminal reading)−1.
Western blotting
Equal amount of protein samples resolved on SDS-polyacrylamide gels and were transferred on the methanol-activated PVDF membranes, followed by blocking with 5% dry milk for 1 h. The membranes were probed with a primary antibody raised against human claudin-5 (1:500, β-actin, 1:300, Sigma, Dorset, UK) and subsequently washed with the wash buffer 1X Tris-buffered saline, 0.1% Tween 20. The membranes were then probed with species-specific infrared dye conjugated appropriate secondary antibodies (goat anti-rabbit, cat no 926-32211; 1: 30,000; Biosciences, Cambridge, UK) for 1 hour and washed to remove excessive antibodies to increase the signal intensities. The protein bands were visualized by Odyssey infrared imaging system (LICOR, Cambridge, UK). The intensities of the bands were then studied and calculated across different treatment groups using the imaging software after normalization against the loading control β-actin.
Cytokines
TNF-α and IL-1β levels were studied using human TNF-α DuoSet DY210 ELISA and human IL-1β/IL-1F2 DuoSet DY008 ELISA, (R&D systems, Abingdon, UK). 96-well plates coated with 100 μl of capture antibody was incubated overnight. On the following day, the plates were rinsed 3 times with wash buffer and blocked by the reagent diluent for 2 h, followed by addition of the detection antibody for further 2 h. Following this, streptavidin-HRP was added and incubated for 20 minutes. Lastly, 50 μl of stop solution added and to determine the optical density, set to 450 nm (FLUOstar Omega multiplate-microplate reader, BMG LABTECH, Aylesbury, UK)
Caspase-3/7
Caspase-3/7 assay was performed using Apo-ONE® homogeneous caspase-3/7 (cat no. G7790; Promega, UK). Briefly, cells grown to ~95% confluence in 96-well plates were subjected to experimental conditions. Following treatments, 100 μl of media from each well was substituted with caspase-3/7 assay buffer containing the non-flourescent caspase substrate rhodamine 110, bis-(N-CBZ-Laspartyl- L-glutamyl-L-valyl-L-aspartic acid amide; Z-DEVD-R110) to initiate reaction. The 96-well plates were then frozen at -80°C overnight. On the following day, plates were thawed on plate shaker for 2 h before reading the fluorescence. The cleavage of the non-fluorescent caspase substrate by caspase-3/7 produced the fluorescent rhodamine-110, read at excitation wavelength of 485 nm and emission of 520 nm. Blanks were subtracted from reading prior to normalizing activity against mg protein.
TUNEL staining
TUNEL staining was performed using a DeadEndTM Colourimetric TUNEL system detection kit (cat.no. G7130, Promega, Southampton, UK). Briefly, cells following treatments, were permeabilised before equilibrating in a buffer containing potassium cacodylate (200 mmol/L), Tris-HCL (25 mmol/l), dithiotheeitol (0.2 mmol/L), BSA (250 mg/L) and cobalt chloride (2.5 mmol/L) for 10 min. The coverslips were then incubated with recombinant terminal deoxynucleotidyl transferase (rTdT) reaction mix containing equilibrium buffer (98 μl), biotinylated nucleotide mix (1 μl) and rTDT enzyme (1 μl) for 60 min to allow end-labeling to occur. The reaction was stopped by immersing coverslips in 20 × SSC buffer before blocking and staining them with 0.3% H2O2 and horseradish peroxide-labelled streptavidin (500 mg/L; 1:500, Promega, Southampton, UK), respectively. TUNEL-positive cells were visualized by diaminobenzidine, which gave dark brown staining and counted TUNEL-positive versus total from 3 different fields per coverslip before calculating mean values for these areas.
Statistical analysis
Data are presented as mean ± SEM. Statistical analysis was conducted using the SPSS statistics 20.0 software package. Mean values were compared by student’s two-tailed t-test or one-way ANOVA, where appropriate, followed by Dunnett’s post-hoc testing. P<0.05 was considered significant.
Results
Effects of hypothermia on OGD associated IL-1β secretions
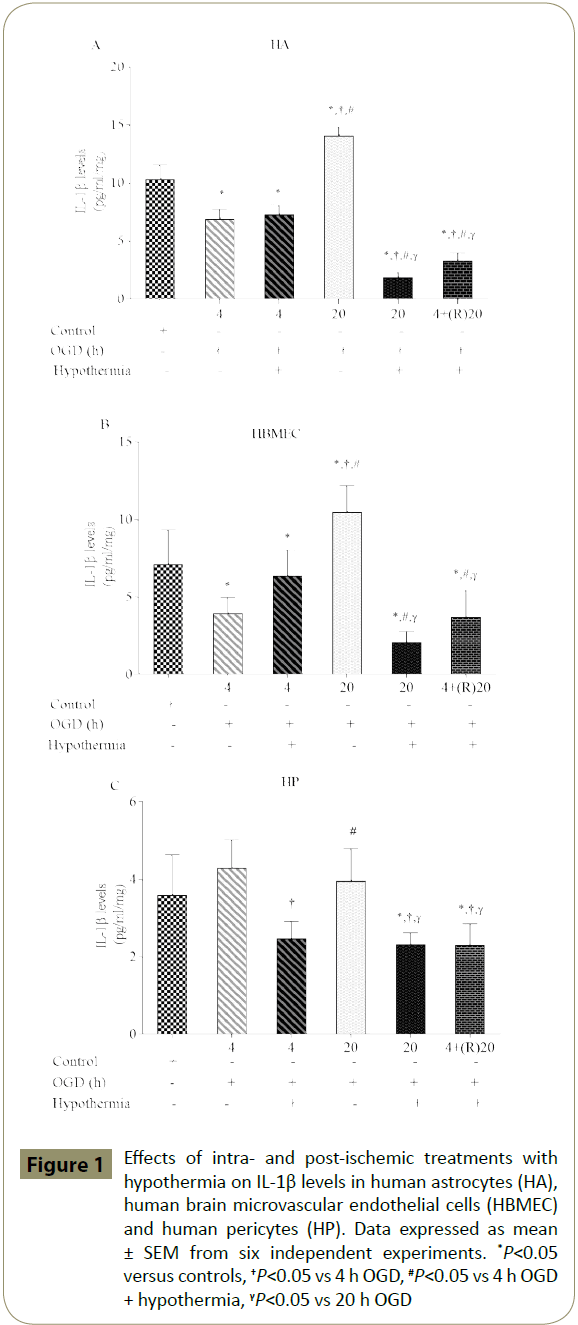
Exposure of cells to shorter periods of OGD (4 h) significantly decreased IL-1β levels in HA and HBMEC compared to the respective control groups. However, as expected treatments with longer periods of OGD alone (20 h) significantly increased IL-1β levels in both cells. Interestingly, levels of IL-1β were not affected in any way in HP by different periods of OGD. Co-exposure of cells to 4 h of OGD plus hypothermia did not suppress IL-1β in HA and HBMEC while significantly diminishing it in HP compared to OGD alone group. Longer application of hypothermia significantly suppressed IL-1β levels in all three cell lines compared to controls; a similar effect was also obtained by post-ischaemic application of hypothermia for 20 h (Figure 1A-C).
Figure 1: Effects of intra- and post-ischemic treatments with hypothermia on IL-1β levels in human astrocytes (HA), human brain microvascular endothelial cells (HBMEC) and human pericytes (HP). Data expressed as mean ± SEM from six independent experiments. *P<0.05 versus controls, †P<0.05 vs 4 h OGD, #P<0.05 vs 4 h OGD + hypothermia, γP<0.05 vs 20 h OGD
Effects of hypothermia on OGD associated TNF-α secretion
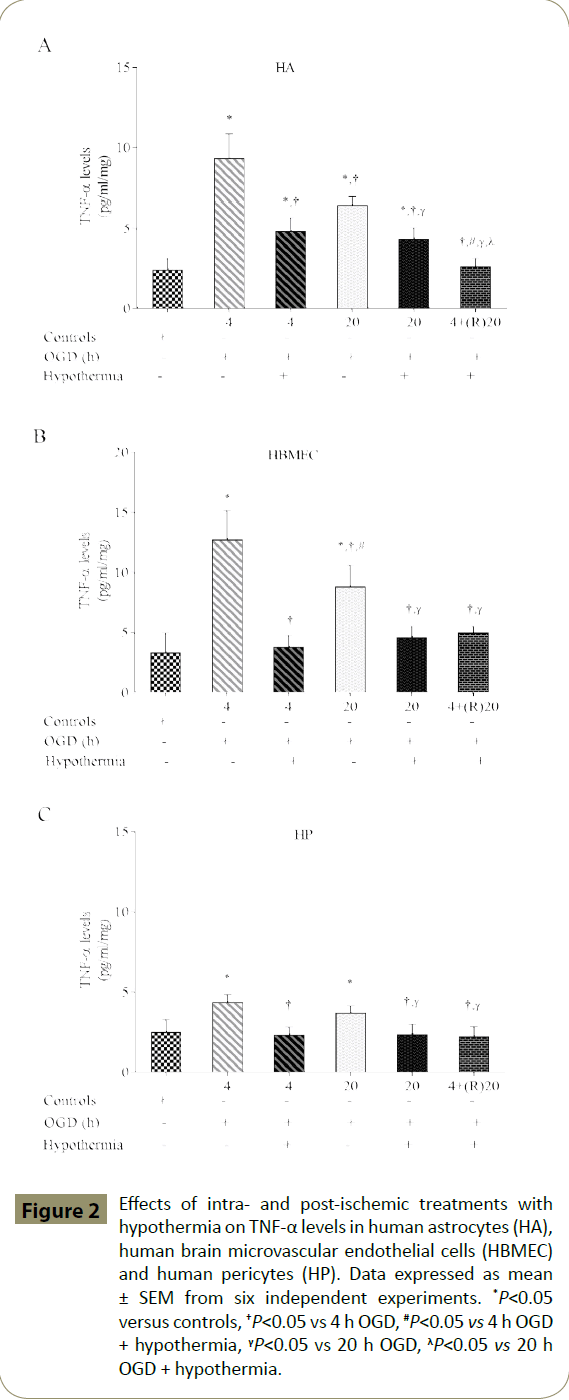
Exposure of cells to shorter periods of OGD (4 h) significantly increased TNF-α level in all three cell lines compared to the respective control groups. In addition, significant upsurge was also documented with longer OGD (20 h) duration. Co-exposure of cells to both 4 and 20 h of OGD plus hypothermia significantly decreased TNF-α levels compared to the respective OGD alone groups. Post-ischaemic exposure to hypothermia for 20 h also decreased TNF-α levels compared to intra-ischaemic OGD alone groups (Figure 2A-C).
Figure 2: Effects of intra- and post-ischemic treatments with hypothermia on TNF-α levels in human astrocytes (HA), human brain microvascular endothelial cells (HBMEC) and human pericytes (HP). Data expressed as mean ± SEM from six independent experiments. *P<0.05 versus controls, †P<0.05 vs 4 h OGD, #P<0.05 vs 4 h OGD + hypothermia, γP<0.05 vs 20 h OGD, λP<0.05 vs 20 h OGD + hypothermia.
Effects of hypothermia on the integrity and function of the in vitro cerebral barrier
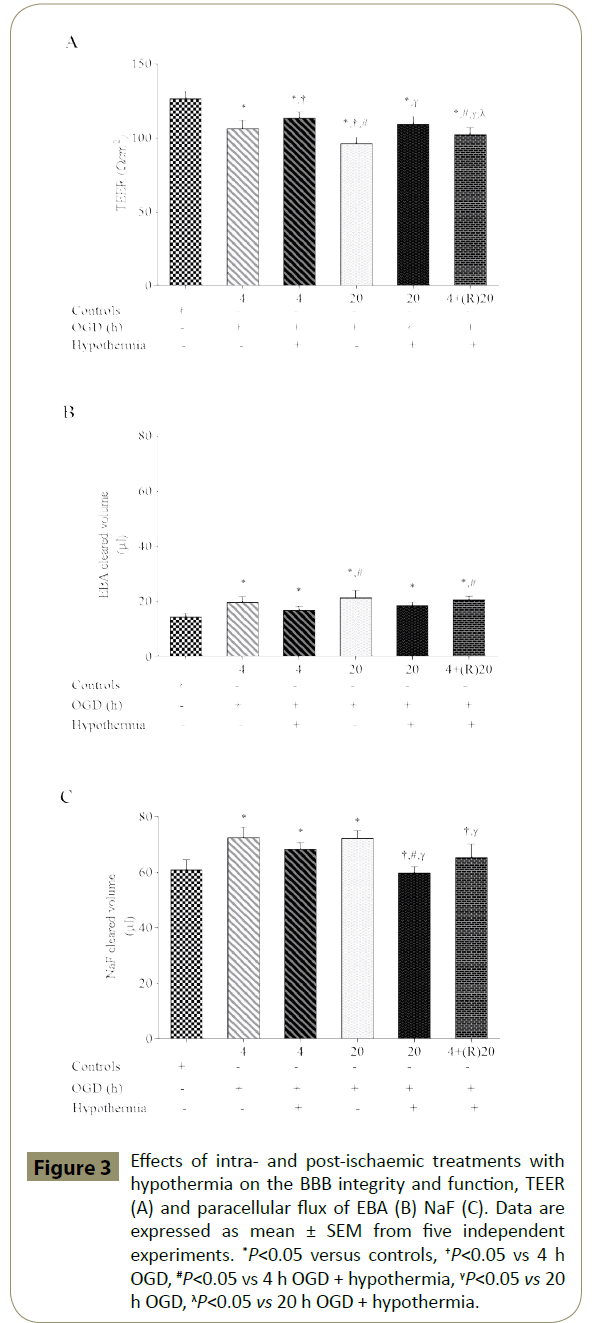
Exposure of triple cultures to both shorter (4 h) or longer (20 h) periods of OGD led to significant impairment of the BBB integrity and function as ascertained by significant decrease in TEER and concomitant increases in paracellular flux of EBA and NaF across the barrier, when compared to respective control groups. Coexposure to OGD plus hypothermia improved barrier integrity and function compared to OGD alone groups but failed to normalise the readings. Post-ischaemic application of hypothermia did not attenuate the impact of OGD on barrier integrity or function (Figure 3A-3C).
Figure 3: Effects of intra- and post-ischaemic treatments with hypothermia on the BBB integrity and function, TEER (A) and paracellular flux of EBA (B) NaF (C). Data are expressed as mean ± SEM from five independent experiments. *P<0.05 versus controls, †P<0.05 vs 4 h OGD, #P<0.05 vs 4 h OGD + hypothermia, γP<0.05 vs 20 h OGD, λP<0.05 vs 20 h OGD + hypothermia.
Effects of hypothermia on claudin-5 protein expression
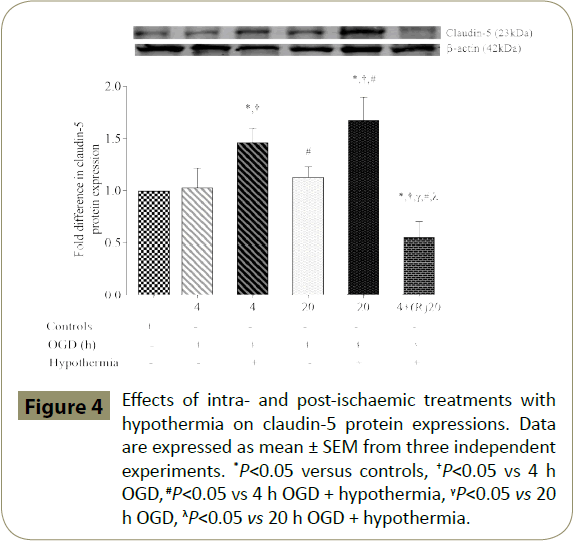
Exposure of HBMEC to both 4 and 20 h of OGD resulted in a slight but insignificant increase in claudin-5 protein expression compared to the controls. Application of hypothermia during the ischaemic episodes significantly increased the protein expression in controls and cells subjected to OGD alone. Postischaemic application of hypothermia (20 h) caused a significant fall in claudin-5 expression when compared to controls and intraischaemic treatment groups (Figure 4).
Figure 4: Effects of intra- and post-ischaemic treatments with hypothermia on claudin-5 protein expressions. Data are expressed as mean ± SEM from three independent experiments. *P<0.05 versus controls, †P<0.05 vs 4 h OGD, #P<0.05 vs 4 h OGD + hypothermia, γP<0.05 vs 20 h OGD, λP<0.05 vs 20 h OGD + hypothermia.
Effects of OGD on DNA fragmentation in the absence or presence of hypothermia
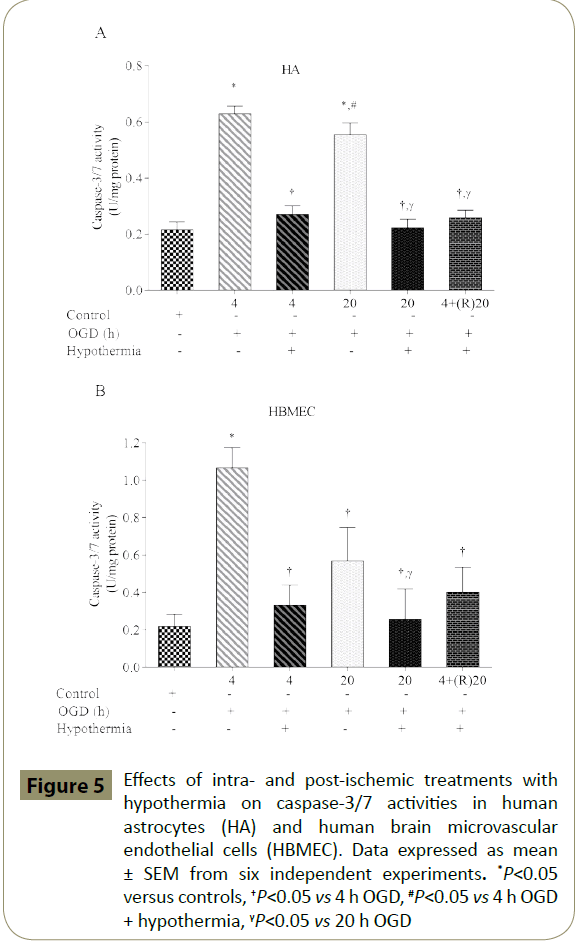
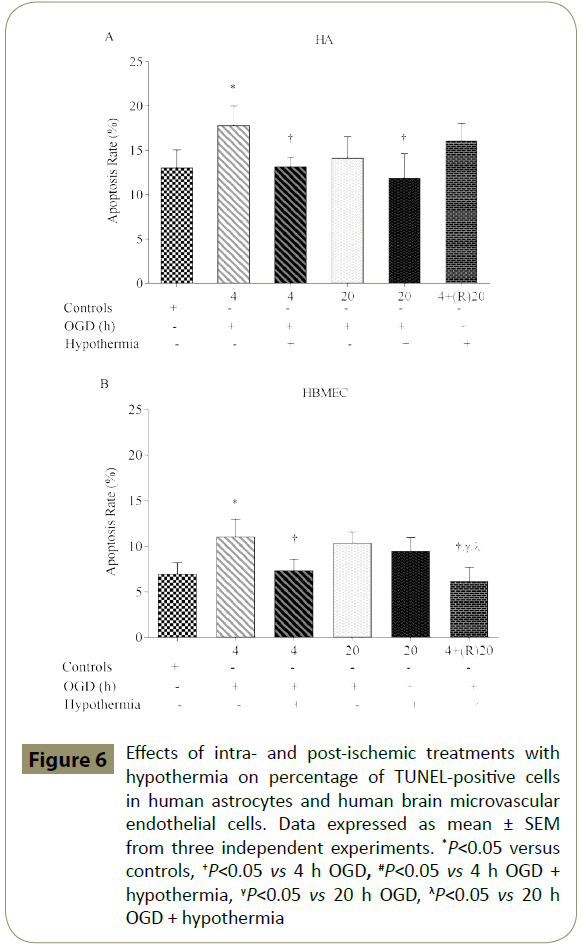
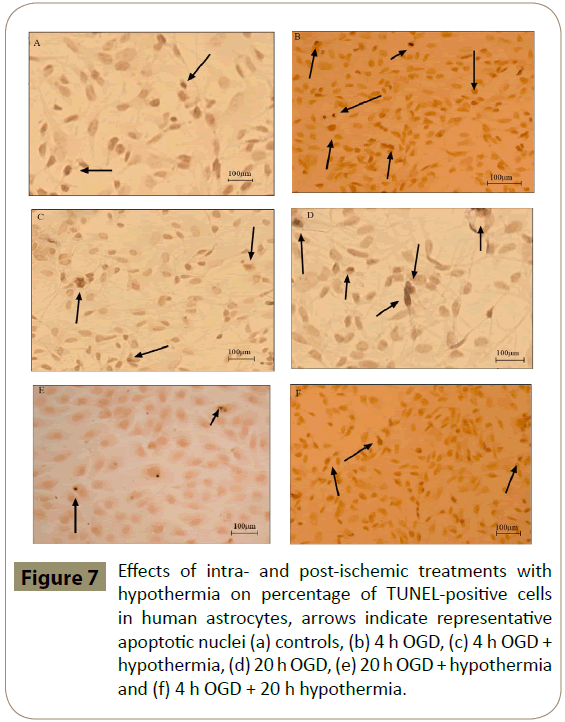
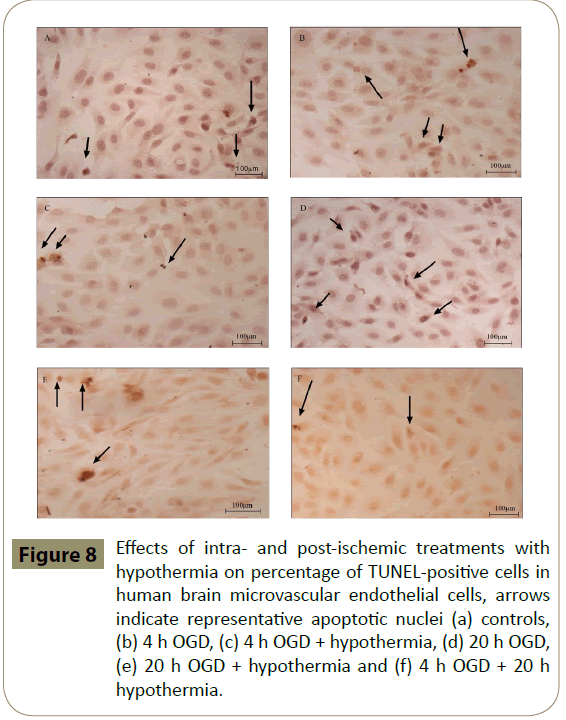
HA and HBMEC exposed to 4 h, but not 20 h, of OGD showed significantly higher percentage of TUNEL-positive cells when compared to controls. Co-application of hypothermia with 4 h OGD markedly decreased the number of TUNEL-positive cells compared to the respective OGD alone groups. Interestingly, post-ischaemic treatment with hypothermia diminished TUNELpositive staining only in HBMEC (Figures 6-8)
Figure 5: Effects of intra- and post-ischemic treatments with hypothermia on caspase-3/7 activities in human astrocytes (HA) and human brain microvascular endothelial cells (HBMEC). Data expressed as mean ± SEM from six independent experiments. *P<0.05 versus controls, †P<0.05 vs 4 h OGD, #P<0.05 vs 4 h OGD + hypothermia, γP<0.05 vs 20 h OGD
Figure 6: Effects of intra- and post-ischemic treatments with hypothermia on percentage of TUNEL-positive cells in human astrocytes and human brain microvascular endothelial cells. Data expressed as mean ± SEM from three independent experiments. *P<0.05 versus controls, †P<0.05 vs 4 h OGD, #P<0.05 vs 4 h OGD + hypothermia, γP<0.05 vs 20 h OGD, λP<0.05 vs 20 h OGD + hypothermia
Figure 7: Effects of intra- and post-ischemic treatments with hypothermia on percentage of TUNEL-positive cells in human astrocytes, arrows indicate representative apoptotic nuclei (a) controls, (b) 4 h OGD, (c) 4 h OGD + hypothermia, (d) 20 h OGD, (e) 20 h OGD + hypothermia and (f) 4 h OGD + 20 h hypothermia.
Figure 8: Effects of intra- and post-ischemic treatments with hypothermia on percentage of TUNEL-positive cells in human brain microvascular endothelial cells, arrows indicate representative apoptotic nuclei (a) controls, (b) 4 h OGD, (c) 4 h OGD + hypothermia, (d) 20 h OGD, (e) 20 h OGD + hypothermia and (f) 4 h OGD + 20 h hypothermia.
Discussion
The BBB is a complex barrier that effectively prevents entry of circulating molecules into the brain parenchyma [22]. However, in patients with ischaemic stroke, the integrity of this specific barrier is compromised. Acute inflammatory reactions accompanied by exaggerated release of pro-inflammatory cytokines are known to significantly contribute to the initiation and progression of oedema [8,23,24]. Rapid revascularisation of the ischaemic brain by recombinant tissue-type plasminogen (r-tPA) continues to be the main medical therapy in the management of acute ischaemic stroke [25]. However, endovascular therapy has now been proven to be an efficacious treatment option for acute ischaemic stroke [26,27]. In fact, thrombectomy with stent retrievers is now recommended as the standard treatment for patients with a proximal large vessel occlusion in the anterior circulation [28]. Even so, all eligible patients continue to receive intravenous r-tPA as standard of care [29]. Given these and the higher risk of intracranial haemorrhage with late thrombolysis, detection of novel therapeutic agents and approaches remains of crucial importance. Therapeutic hypothermia, defined as core body temperature ≤35°C, has long been regarded as a neuroprotective therapy in other ischaemic settings like cardiac arrest [30]. Interestingly, the current data regarding the time window in which therapeutic hypothermia can be applied after an acute ischaemic attack and those regarding the degree and optimal duration of hypothermia are rather limited. In animal models of transient cerebral ischaemia, temperatures between 31-35°C have proven highest efficacy in infarct size reduction [31] while current guidelines for neuroprotective target temperature in patients with cardiac arrest recommend 32-34°C [32]. Taken together, these findings suggest that temperatures between 32-35°C may also be feasible target in patients with ischaemic stroke. Considering that neurovascular insult following an acute ischaemic attack occurs over hours to days, it is presumed that longer durations of hypothermia may lead to better neurological outcomes [33]. However, the extended treatments may also trigger various side effects such as rebound hyperkalaemia, unwanted shivering and infection [32].
Elevated release of pro-inflammatory cytokines like TNF-α during or after an ischaemic attack may play a critical role in compromising the BBB integrity or aggravating the extent of damage [1,8]. Hence, this study examined the correlation between two key inflammatory cytokines, namely TNF-α and IL-1β and the level of BBB breakdown during intra- and postischaemic phases in the absence or presence of hypothermia.
First, to address whether the release of these cytokines may be dictated by the duration of ischaemic injury, HBMEC, astrocytes and pericytes were exposed to OGD for 4 or 20 h. Concomitant exposure of cells to hypothermia in parallel experiments revealed the therapeutic impact of hypothermia. In accordance with the well-documented late release pattern of IL-1β, this study also shows selective increases in IL-1β levels in HBMEC and astrocytes after 20 h of exposure to OGD [34]. In contrast to IL-1β, TNF-α constitutes a pleotropic cytokine with both neurovascular disruptive and protective roles [7]. TNF-α activates glia whereby endorses its own production in addition to many other mediators ultimately leading to oxidative stress, cytoskeletal reorganisation and basement membrane degradation stemming from matrix metalloproteases (MMP) activation [35]. Downregulation of both IL-1β and TNF-α in the current study with hypothermia in all BBBrelated cells during intra- and post-ischaemic phases confirms the potential barrier-protective effects of this treatment regimen.
Pro-inflammatory cytokines, notably TNF-α accounts for apoptosis of endothelial cells and therefore facilitates infiltration of immune cells into the brain parenchyma through openings in the BBB [36]. This process is further augmented by the ischaemia-mediated excessive release or synthesis of MMPs and proteases that collectively digest basement membrane proteins to compromise extracellular matrix [37]. As endothelial cells express and retain the tight junction proteins only when co-cultured with astrocytes (and possibly pericytes), the use of triple culture model in the current study was of pivotal importance [38]. Similar to in vivo settings, exposure of this model to ischaemic conditions perturbed BBB integrity and function as evidenced by marked decreases in TEER and simultaneous increases in paracellular flux, respectively. However, concomitant application of therapeutic hypothermia during intra- and post-ischaemic settings effectively negated the deleterious effects of ischaemic injury on BBB integrity and function, confirmed by increases in TEER and decreases in flux of high (EBA) and low (NaF) molecular weight permeability markers across the barrier.
Changes in the expression and localisation of tight junction proteins may represent one of the main causes of BBB hyperpermeability during an ischaemic insult. Indeed, impairments in BBB integrity and function during reperfusion injury have previously been attributed to the markedly reduced expressions of key junctional proteins, namely occludin, claudin-5 and ZO-1 which may partly be due to enhanced release of TNF-α and IL-1β [39]. Indeed, exaggerated presence of TNF-α has been shown to account for decreases observed in occludin and claudin-5 expressions in a mouse model of stroke [13,40]. In this context, the current study shows that treatments with hypothermia during an ischaemic episode can effectively increase claudin-5 protein expressions and may help maintain appropriate barrier function. In support of our findings, exposure of experimental stroke models to early hypothermia has also been shown to help preserve tight junction protein expressions [41,42].
Apoptosis emerging from a sudden decline in oxygen and nutrient levels during acute phase of the stroke also contributes to BBB dysfunction. Indeed, exposure of all BBB-forming cells to short periods of OGD i.e. 30 min has been shown to potentiate calcium influx and pro-apoptotic caspase-3/7 activities, key phenomena in elevated rates of cellular death [43]. Namely, inhibition of caspase activities has previously been shown to rescue neurons from ischaemia-induced apoptosis [44]. As expected, activation of caspase-3/7 by TNF-α and/or IL-1β has previously been linked with tight junction dissolution and endothelial cell apoptosis [13,36,45]. In the current study, treatment of HBMEC and astrocytes with hypothermia significantly reduced the levels of DNA fragmentation and pro-apoptotic caspase-3/7 activities induced by intra- or postischaemic injuries and hence contributed to the maintenance of barrier function.
Conclusion
Release of pro-inflammatory cytokines TNF-α and IL-1β during an acute ischaemic injury or immediately afterwards appears to perturb BBB integrity through dissolution of tight junctional unity and stimulation of endothelial cell and astrocyte apoptosis. Application of hypothermia as a therapeutic regimen during and post-ischaemic phases may protect BBB integrity and function by negating the aforementioned deleterious effects of ischaemic injury.
References
- Abdullah Z, Bayraktutan U (2016) Suppression of PKC-alpha attenuates TNF-alpha-evoked cerebral barrier breakdown via regulations of MMP-2 and plasminogen-plasmin system. BiochimBiophysActa 1862: 1354-1366.
- Ballabh P, Braun A, Nedergaard M (2004)The blood-brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis 16: 1-13.
- Hayashi K, Nakao S, Nakaoke R, Nakagawa S, Kitagawa N, et al. (2004) Effects of hypoxia on endothelial/pericytic co-culture model of the blood-brain barrier. RegulPept 123: 77-83.
- Gibson CL, Srivastava K, Sprigg N, Bath PM, Bayraktutan U (2014) Inhibition of Rho-kinase protects cerebral barrier from ischaemia-evoked injury through modulations of endothelial cell oxidative stress and tight junctions. J Neurochem 129: 816-826.
- Hayashi M, Luo Y, Laning J, Strieter RM, Dorf ME (1995) Production and function of monocyte chemoattractant protein-1 and other beta-chemokines in murine glial cells. J Neuroimmunol 60: 143-150.
- Gutierrez EG, Banks WA, Kastin AJ (1993) Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol 47: 169-176.
- Abdullah Z, Bayraktutan U (2014) NADPH oxidase mediates TNF-alpha-evoked in vitro brain barrier dysfunction: roles of apoptosis and time. Mol Cell Neurosci 61: 72-84.
- Abdullah Z, Rakkar K, Bath PM, Bayraktutan U (2015) Inhibition of TNF-alpha protects in vitro brain barrier from ischaemic damage. MolCell Neurosci 69:65-79.
- Wang Q, Tang XN, Yenari MA (2007)The inflammatory response in stroke. J Neuroimmunol 184: 53-68.
- Simi A, Tsakiri N, Wang P, Rothwell NJ (2007) Interleukin-1 and inflammatory neurodegeneration. BiochemSoc Trans 35: 1122-1126.
- Janardhan V, Qureshi AI (2004) Mechanisms of ischemic brain injury. CurrCardiol Rep 6: 117-123.
- Ding HF, Fisher DE (2001) p53, caspase 8, and regulation of apoptosis after ionizing radiation. J PediatrHematolOncol 23: 185-188.
- Lopez-Ramirez MA, Fischer R, Torres-Badillo CC, Davies HA, Logan K, et al. (2012) Role of caspases in cytokine-induced barrier breakdown in human brain endothelial cells. J Immunol 189: 3130-3139.
- Hawkins BT, Davis TP (2005)The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev 57: 173-185.
- McCole DF (2013) Phosphatase regulation of intercellular junctions. Tissue Barriers 2013 1): e26713.
- Furuse M, Fujita K, Hiiragi T, Fujimoto K, Tsukita S (1998) Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol 141: 1539-1550.
- Khatri R, McKinney, Swenson B, Janardhan V (2012) Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 79: S52-57.
- Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, et al. (2013) Investigators, A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 368: 914-923.
- Guluma KZ, Hemmen TM, Olsen SE, Rapp KS, Lyden PD (2006) A trial of therapeutic hypothermia via endovascular approach in awake patients with acute ischemic stroke: methodology. AcadEmerg Med 13: 820-827.
- Small DL, Morley P, Buchan AM (1999) Biology of ischemic cerebral cell death. ProgCardiovasc Dis 42: 185-207.
- Shao B, Bayraktutan U (2014) Hyperglycaemia promotes human brain microvascular endothelial cell apoptosis via induction of protein kinase C and prooxidant enzyme NADPH oxidase. Redox Biol 2: 694-701.
- Bazzoni G, Martinez-Estrada OM, Orsenigo F, Cordenonsi M, Citi S, et al. (2000) Interaction of junctional adhesion molecule with the tight junction components ZO-1, cingulin, and occludin. J BiolChem 275: 20520-20526.
- Ferrarese C, Mascarucci P, Zoia C, Cavarretta R, Frigo M, et al. (1999) Increased cytokine release from peripheral blood cells after acute stroke. J Cereb Blood Flow Metab 19: 1004-1009.
- Kassner A, Merali Z (2015) Assessment of Blood-Brain Barrier Disruption in Stroke. Stroke 46: 3310-3315.
- Goyal M, Yu AY, Menon BK, Dippel DW, Hacke W, et al. (2016) Endovascular Therapy in Acute Ischemic Stroke: Challenges and Transition From Trials to Bedside. Stroke 47: 548-553.
- Berkhemer OA, Fransen P, Beumer D, van den Berg LA, Lingsma HF, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 372: 11-20.
- Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 372: 1009-1018.
- Powers WJ, Derdeyn CP, Biller J, Coffey CS, Hoh BL, et al. (2015) American Heart Association Stroke Council. 2015 American Heart Association/American Stroke Association Focused Update of the 2013 Guidelines for the Early Management of Patients With Acute Ischemic Stroke Regarding Endovascular Treatment: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 46: 3020-3035.
- Mayank G, Yu YX, Menon BK, Dippel DWJ, Hacke W, et al. (2016) Endovascular Therapy in Acute Ischemic Stroke Challenges and Transition From Trials to Bedside. Stroke47:548-553.
- Trescher K, Gleiss A, Boxleitner M, Dietl W, Kassal H, et al. (2017) Short-term clinical outcomes for intermittent cold versus intermittent warm blood cardioplegia in 2200 adult cardiac surgery patients. J CardiovascSurg (Torino) 58: 105-112.
- van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR (2007) Hypothermia in animal models of acute ischaemic stroke: a systematic review and meta-analysis. Brain 130: 3063-3074.
- Loach B, Bayraktutan U (2017) Hypothermia: a Therapeutic Option in Management of Stroke. Stroke Resarch& Therapy 2:3.
- Dirnagl U (2012) Pathobiology of injury after stroke: the neurovascular unit and beyond. Ann N Y AcadSci 1268: 21-25.
- Legos JJ, Whitmore RG, Erhardt JA, Parsons AA, Tuma RF, et al. (2000) Quantitative changes in interleukin proteins following focal stroke in the rat. Neurosci Lett 282: 189-192.
- Lambertsen KL, Clausen BH, Babcock AA, Gregersen R, Fenger C, et al. (2009) Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J Neurosci 29: 1319-1330.
- Sriram K, O'Callaghan JP (2007) Divergent roles for tumor necrosis factor-alpha in the brain. J NeuroimmunePharmacol 2: 140-153.
- Kim JY, Kawabori M, Yenari MA (2014)Innate inflammatory responses in stroke: mechanisms and potential therapeutic targets. Curr Med Chem 21: 2076-2097.
- Rakkar K, Srivastava K, Bayraktutan U (2014) Attenuation of urokinase activity during experimental ischaemia protects the cerebral barrier from damage through regulation of matrix metalloproteinase-2 and NAD(P)H oxidase. Eur J Neurosci 39: 2119-2128.
- Jiao H, Wang Z, Liu Y, Wang P, Xue Y (2011) Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult. J MolNeurosci 44: 130-139.
- Li GZ, Wang ZH, Cui W, Fu JL, Wang YR, et al. (2012)Tumor necrosis factor alpha increases intestinal permeability in mice with fulminant hepatic failure. World J Gastroenterol 18: 5042-5050.
- Sun H, Tang Y, Guan X, Li L, Wang D (2013)Effects of selective hypothermia on blood-brain barrier integrity and tight junction protein expression levels after intracerebral hemorrhage in rats. BiolChem 394: 1317-1324.
- Knowland D, Arac A, Sekiguchi KJ, Hsu M, Lutz SE, et al. (2014) Stepwise recruitment of transcellular and paracellular pathways underlies blood-brain barrier breakdown in stroke. Neuron82: 603-617.
- DeGraba TJ, Ostrow PT, Strong RA, Earls RM, Zong ZJ, et al. (1992) Temporal relation of calcium-calmodulin binding and neuronal damage after global ischemia in rats. Stroke 23: 876-882.
- Maier JK, Lahoua Z,Gendron NH, Fetni R, Johnston A, et al. (2002) The neuronal apoptosis inhibitory protein is a direct inhibitor of caspases 3 and 7. J Neurosci 22: 2035-2043.
- Yuan J (2009) Neuroprotective strategies targeting apoptotic and necrotic cell death for stroke. Apoptosis 14: 469-477.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences